Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
A Spanish research group has developed a new non-noble metal-based catalyst to remove the emission of hydrocarbons (HC) in internal combustion engines. A patent for this technology has been applied for. The material, which has been developed at laboratory scale, can act as a HC trap and as an oxidation catalyst during the whole cold start cycle of the engine.
Researchers are looking for partners to license the technology. They are also open to establish further joint development projects.

Combustion gases (carbon dioxide (CO2) and water) and other by-products resulting from incomplete combustion (carbon monoxide (CO), unburned hydrocarbons and other polluting gases (e.g., nitrogen oxides, NOx) are generated in internal combustion engines when fuels are burnt with air. For this reason, exhaust gases have to be cleaned prior to be released into the atmosphere in order to comply with the environmental regulations stipulated by the different government policies.
It is well-known the treatment of exhaust gases coming from internal combustion engines to turn toxic compounds (hydrocarbons (HC), CO, NOx and even particles (e.g., soot in the case of diesel engines)) into innocuous compounds such us water, CO2 and nitrogen. This procedure consists of putting in touch the exhaust gases stream with a control system of such pollutants called catalytic converter. This system usually includes catalysts that, unfortunately, are not only active during the whole driving cycle but also do not start to operate until they reach temperatures about 200ºC. Then, HC emissions in internal combustion engines are mainly produced during the cold start.
The research group has developed a catalytic trap based on an adsorbent material to decrease HC emissions in exhaust gases of internal combustion engines, and specially, to minimize the emissions of these compounds during an engine cold start. This system is free of noble metals and includes one or several layers of a molecular sieve containing one or several transition metals (Cu, Fe, Zn, Co or Ni).
Basically, the catalytic trap bed is composed of a zeolite with a Si/Al ratio between 10 and 20. The zeolite is partially interchanged with cations of one or several non-noble metals. In order to achieve an optimum performance of the catalytic trap, these metals should be interchanged in the internal zeolite structure, and never on its external surface. In this way, the outflow of the exhaust gases passes through the catalytic trap bed to adsorb the HC at low temperatures.
The material has been developed at laboratory scale. Different compositions of this material have been tested with simulated streams of internal combustion engines (cold starts). As a result, the material is able to reduce HC emissions in internal combustion engines operating with both mixtures almost stoichiometric and low fuel mixtures.
The main difference between this invention and other existing materials is that this catalytic trap avoids any element or additional layer composed of an oxidation catalyst based on noble metals. Consequently, HC emissions could be totally removed through a single bed without using high-cost materials (noble metals) or further stream treatments. This fact allows the catalytic trap to be placed in any position according to the different control systems employed for decreasing other pollutant emissions existing in the gases stream, since the total elimination of HC takes place on the catalytic trap.
Thus, this technology development results in a solid material where coexists metal(s) and protons in an optimum ratio inside of the zeolite channels, leading to a system that can act as a HC trap and as an oxidation catalyst in only a single bed, during the whole cold start cycle.
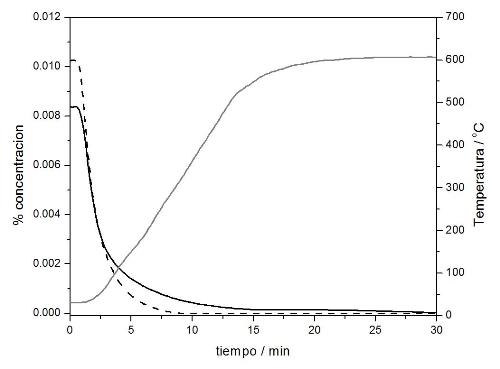
The following figure shows, as an example, the behaviour of one of the catalytic traps designed during a combustion engine cold start simulation (exhaust simulation gas stream: 100 ppmv propene, 87 ppmv toluene, 1.0%O2, 10% H2O and balance Ar). The continuous line shows the concentration of Toluene, the discontinuous line shows the concentration of propene and the temperature ramp is drawn in grey colour:
MAIN ADVANTAGES OF THE TECHNOLOGY
The most important advantages in comparison to other catalytic systems are:
At high temperatures, this material is able to carry out total oxidation of both hydrocarbons retained by the catalytic trap and those present in the exhaust gas stream. Consequently, the resulting gas stream released to the atmosphere is innocuous in hydrocarbons.
INNOVATIVE ASPECTS
The main innovative aspect of this catalytic trap is that the adsorbent material can capture the hydrocarbons in the cold start of the engine and oxidize gases during its warmed-up operating conditions without using noble metals, which are frequently used as oxidation catalyst.
Development phase (laboratory tested): The material has been developed at laboratory scale. Different R&D studies and tests have been performed with simulated exhaust gases from engines (cold start). Different compositions of the catalyst have been tested as well.
Other potential domains of application are:
Engineering, Robotics and Automation
Chemical Technology
Transport and Automotive
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959





