Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Molecular Nanotechnology Laboratory of the University of Alicante, together with the Federal University of Rio de Janeiro, has developed a new class of hybrid catalysts that combine a catalytic phase (zeolites) with a heating phase (silicon carbide nanoparticles) embedded inside. This unique integration enables much more efficient heat transfer during chemical reactions, significantly accelerating them and reducing energy consumption by up to 60%. Faster and more uniform heating results in a higher conversion rate of the reactants, and the combination of micro- and mesoporous structures favours the formation of specific products, improving the selectivity of the reaction.
This technology can be applied in a wide variety of industrial processes, such as chemical (catalytic cracking, etc.), pharmaceutical and energy (biomass processing and CO2 conversion).
It is looking for companies interested in acquiring this technology for its commercial exploitation through patent license agreements.

Many catalytic solid materials, such as zeolites, are widely used in different industrial processes. In the context of catalysed industrial chemical reactions, microwave and/or induction heating is commonly used to accelerate such reactions. However, these catalytic solids usually have a low microwave absorption and induction capacity.
For this reason, additives with high microwave absorption and induction power such as silicon carbide (SiC) are blended with the solid catalysts. An intimate contact between the two phases is required, as this contact largely determines the effectiveness of the additive in heating the catalytic phase.
To date, several different solutions are known in which the heating material and the catalytic material are simply mixed. However, there is still a need to provide alternative hybrid catalytic materials in which the catalyst can be improved by the addition of microwave adsorbent additives, so that heat transfer can be achieved more efficiently in the context of catalysed industrial chemical reactions.
In order to solve the above need, the present invention describes the integration of a heating phase incorporated within a catalytic phase, such that the heating phase is completely contained within the catalytic phase. An example of such a hybrid material is obtained by synthesising zeolite in the presence of SiC nanoparticles, resulting in a composite material consisting of zeolite crystals trapping SiC nanoparticles (see Figure 1).
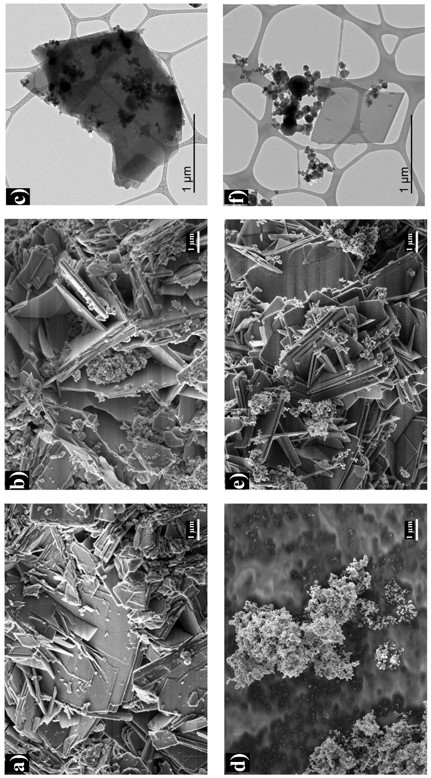
Figure 1: SEM images of a) FER, b) FER@SiC, c) SiC, d) FER/SiC; and TEM images of e) FER@SiC and f) FER/SiC.
The present invention relates to a composition comprising a catalytic phase and a heating phase, wherein the heating phase is embedded within the catalytic phase.
The catalytic phase is a material which is selected from a zeolite (zeolites are microporous crystalline aluminosilicate materials) or a material with similar structure and function to zeolites (isomorphous substitution of zeolites aluminophosphates and silicoaluminophosphates), a metal oxide (binary, ternary or mixed) or an organometallic structure (porous polymers consisting of metal groups coordinated with organic ligands to form one, two or three-dimensional structures).
The catalytic material is modified with at least one functional group which can be selected from among the following: amine, phosphine, carbonyl, carboxylic, aromatic, alcohol, aliphatic chains and combinations thereof, cationic, anionic and/or neutral polymers, metal groups, metal complexes and enzymes.
The heating phase is selected from: a semiconducting material, a magnetic solid or alloy, a ferromagnetic compound, a dielectric material, an intrinsically conductive polymer or a biomass-derived compound.
The heating phase consists of nanoparticles or nanowires in the size range of 1-100 nanometres.
The heating phase can be an amorphous material (solid with an unordered three-dimensional structure, e.g. glass, metallic glass and certain plastics or polymers) or a crystalline material (solid whose atoms, molecules or ions are arranged in an ordered three-dimensional structure forming a crystalline lattice).
The heating phase is a material that exhibits high microwave absorption and/or induction capability and is capable of dissipating the absorbed electromagnetic energy in the form of heat, the value of the tangent of the loss angle δ > 0,5.
The heating phase has a higher microwave and/or induction absorption capacity than the catalytic phase.
The present invention relates to a method for preparing a catalytic composition in which the catalytic phase is synthesised in the presence of the heating phase, such that the heating phase is completely included within the catalytic phase. For this purpose, the heating phase is added to the synthesis mixture, and then the catalytic phase is synthesised. After preparation of the catalytic phase, the heating phase is integrated into the catalytic phase.
The preparation of the catalytic phase can be carried out by any method known in the state of the art, including: hydrothermal, alkaline fusion, alkaline hydrothermal, alkaline leaching, sol-gel, microwave synthesis, ionothermal, solvothermal, ultrasonic energy synthesis, etc.
The catalytic phase and/or the heating phase is coated, functionalised or modified with a polymer, chemical functional group or biomolecule before the catalytic phase is synthesised.
ADVANTAGES OF THE TECHNOLOGY
These new catalysts have the following advantages:
1) They are more efficiently heated.
2) They have a higher catalytic activity than current catalysts, with a 2,5 times higher conversion rate.
3) The main zeolite properties (acidic, textural, structural and morphological characteristics) are not affected by the presence of silicon carbide nanoparticles.
4) No significant blockage of pores due to the presence of nanoparticles.
5) Improved selectivity due to the combination of micro- and mesoporous structures.
6) Significant improvement in performance as a result of efficient heat transfer from nanoparticles to reagents.
7) MARKED REDUCTION IN ENERGY CONSUMPTION (MORE THAN 60%) when comparing conventional zeolites to the materials of the present invention (see Figure 2).
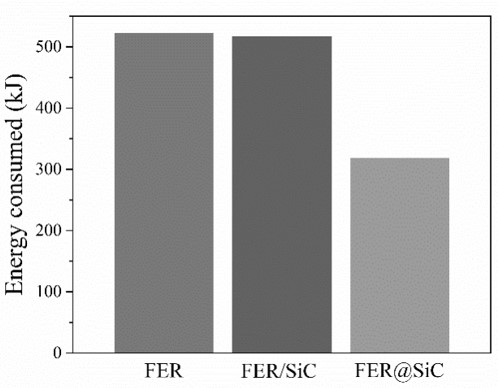
Figure 2: Energy consumed by each catalyst in the isoconversion of 15% benzyl alcohol in the microwave reactor.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The obtained results show that mixing materials with high microwave absorption and/or induction capacity with catalytic materials is not an effective strategy to accelerate the heating of the catalytic material due to poor contact between them. Therefore, it is essential to ensure a close contact between the heating phase and the catalytic phase to achieve an efficient microwave-assisted catalysis. This objective is achieved by the procedure described in the present invention.
The technology is at a stage of maturity corresponding to TRL = 4 (Technology Readiness Level).
The new hybrid catalytic materials have been synthesised at laboratory scale and successfully validated in the Friedel-Crafts alkylation chemical reaction of mesitylene with benzyl alcohol.
Ferrite zeolite crystals (FER) have been synthesised in the presence of silicon carbide (SiC) nanoparticles, resulting in a composite material consisting of FER crystals with SiC nanoparticles embedded inside (FER@SiC). The composite material has been prepared under the same conditions as the pure FER sample, but in the presence of SiC nanoparticles.
Some conclusions obtained in the different tests carried out are shown below:
• The main zeolite properties (acidic, textural, structural and morphological) are not affected by the presence of silicon carbide nanoparticles.
• A striking difference in catalytic activity is observed, with a catalytic performance 2,5 times higher than that of the physical mixture (see Figure 3). This result clearly demonstrates the importance of intimate contact between the heating and catalyst phases, and shows the potential of the technique described here.
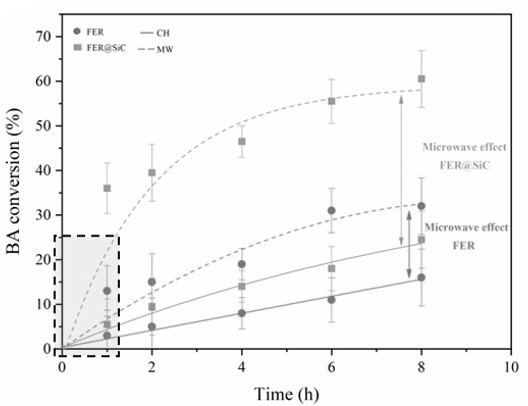
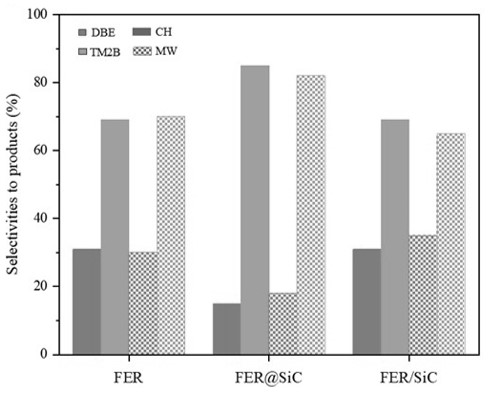
Figure 3: Amount of benzilic acid converted in short times for the three materials, and selectivities towards dibenzyl ether (DBE) and 1,3,5-trimethyl-2-benzylbenzene (TM2B), both in conventional (solid) and microwave (lines) heating for FER, FER@SiC and FER/SiC, with 15% isoconversion in Friedel-Crafts alkylation, respectively.
• Better selectivity due to the combination of micro and mesoporous structures.
• Requires only about 60% of the necessary energy input compared to conventional catalysts.
These results open up new opportunities for the rational design of more efficient zeolite-based catalysts, thus advancing the field of microwave chemistry towards greater attractiveness and practicality.
The present invention belongs to the technical field of catalysed chemical reactions assisted by microwave or induction heating.
Specifically, it concerns catalysts incorporating additives with high microwave and/or induction absorption capacity.
This novel catalytic composition can be effectively used as:
• Adsorbent in drying, purification or separation processes.
• Ion exchanger.
• Catalyst for chemical reactions, including:
o Catalytic cracking.
o Alkylation.
o Acylation.
o Isomerisation.
o Oligomerisation.
o Hydrocracking.
o Hydrotreating.
o Biomass transformation.
o Water Gas Shift reaction (WGS).
o Reverse Water Gas Shift reaction (RWGS).
o Methanol-to-hydrocarbons (MTH) reaction.
o CO2 valorization reaction.
o Condensation reaction.
These new catalysts make it possible to use electrical energy to replace traditional heating in the chemical industry, which is currently carried out by burning fossil fuels (coal, gas and oil), with a more sustainable, cleaner and more efficient electrical system.
We are looking for companies interested in acquiring this technology for commercial exploitation through patent licensing agreements.
Company profile sought:
• Chemical industry.
• Pharmaceutical industry.
• Catalysis companies.
• Energy companies.
The present invention is protected through patent application:
• Patent title: "Hybrid composition comprising a catalytic phase and an embedded heating phase".
• Application number: EP24382634.4.
• Application date: 12th June, 2024.
Materials and Nanotechnology
Chemical Technology

Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




