Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
This invention solves the disadvantages of the methods known so far, since they do not require highly-reactive and/or highly-toxic substances. It can be applied both in solution and on cotton swabs.
The group is looking for companies interested in acquiring this technology for commercial exploitation.

So far, formylindolizines are obtained by classical formilation methods which have the disadvantage of requiring highly-reactive and/or highly-toxic substances such as phosphoryl chloride or butyl lithium. Furthermore, in some cases more than two reagents are required, apart from the substrate to be formylated and the solvent, with the temperature being an important parameter to be controlled either by heating and cooling.
On the other hand, it is essential to have efficient methods for detecting nitrites due to the risk of their accumulation in both physiological and environmental systems. The intake of this anion can have a detrimental effect on the health of mammals, macro-invertebrates and most aquatic organisms. Nitrites, on the one hand, are widely used as preservatives, protecting food from micro-organisms, but, in contrast, under acidic conditions in the stomach, they can give rise to highly carcinogenic compounds (gastric cancer).
Colorimetric methods are the most convenient for the detection of nitrites in water and food, as they are the simplest and most direct ones, usually based on diazotization reactions. However, this test has some disadvantages, such as:
• The need to control the reaction so that it does not decompose the diazonium salt before coupling.
• Narrow working concentration range.
• Long steady-state times.
• Toxicity of the process.
An alternative is that employing nitrosation/nitration reactions; they are simpler, as they eliminate the coupling with the second component, although colour changes are not so evident, especially when it is intended to detect trace amounts, and the resulting products can be highly toxic and dangerous.
Therefore, there is a double need: on one hand, the need to provide new methods for the formylation of indolizines that do not require highly-toxic substances and that are not incompatible with the functional groups of the compounds, and do not require a precise the control of the parameters. On the other hand, there is a need to develop new methods for the detection of nitrites that are fast, simple and efficient in different concentration ranges, and that can be carried out both in solution and in other types of media.
To cover these needs, the ISO of the University of Alicante has developed a reaction of 1,3-disubstituted indolizines with the Eschenmoser salt that allows to obtain not the product of dimethylaminomethylation, but the one of a direct and regioseletive formylation at the position 7 of the indolizine ring. The reaction takes place in acetonitrile at room temperature, in the presence of sodium bicarbonate as a base (see Figure 1).
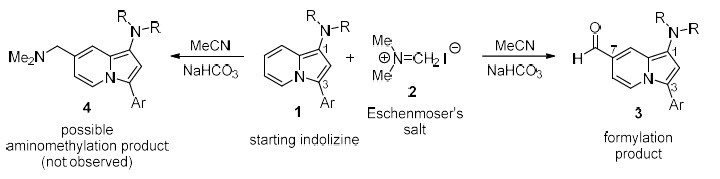
Figure 1: Reaction of an indolizine of general structure (1), substituted at the positions 1 and 3, with the Eschenmoser’s salt (2) in the presence of sodium bicarbonate. The typical product of dimethylaminomethylation (4, not formed) and the formylation product (3, observed) are shown.
The resulting products have shown high selectivity in the detection of nitrite ions compared to other thirteen anions in an acidic medium. The presence of the nitrite ion with this test is manifested in solution with the appearance of a coloration ranging from reddish, more or less intense, to pinkish, depending on the concentration of the nitrite ion, while the rest of the anions present a pale yellow colour or are colorless (see Figure 2).

Figure 2: Nitrite ion selective detection test (NaNO2, reddish solution), against thirteen anions, using a formylindolizine in acidic medium. Salts: KCN, PhCO2K, KF, KCl, KBr, KI, KOAc, K2P4O7, K3PO4, KH2PO4, NaN3, K2S y NaHS. All concentrations are 10–4 M.
The nitrite detection test can be applied in solution (with more intense colouring), as well as on a white support, such as a cotton swab. For lower concentrations of nitrite, the presence of sodium chloride accelerates the appearance of the colour. This test has been successfully applied in the detection of 3 mg L-1 (3 ppm) of sodium nitrite in drinking water, an amount set by the World Health Organisation as a safe limit, as well as in the detection of nitrites as preservatives in various foods (e.g. Frankfurt sausages). Moreover, the test is equally effective in detecting the maximum amount of nitrite in drinking water set by the EPA (1 ppm, 1.45 × 10-5 M).
TECHNOLOGY ADVANTAGES
The main advantages of this technology are the following ones:
• The colour change in the procedure for detecting nitrites is immediate, except in the case of very low concentrations.
• The test can be applied in solution or on cotton swabs at a wider range of concentrations.
• No highly reactive or toxic substances are required.
• It only involves an organic substance at very low concentration 10-4 M.
• It does not require any exhaustive control of the process.
• No apparent generation of residues and/or harmful products.
• The high range of concentrations.
• Selectivity.
INNOVATIVE ASPECTS OF TECHNOLOGY
The present invention describes a novel method of formylation which uses, for the first time, the Eschenmoser salt (solid substance) as a formylation agent, in the presence of sodium bicarbonate at room temperature and which, applied to indolizines, makes it possible to obtain the corresponding carbaldehydes (7-formylindolizines) in a regioselective manner. This is the first formylation reaction at the position 7 of the indolizine ring.
Furthermore, these carbaldehydes have been shown to be selective agents for the rapid, simple and efficient detection of nitrites at low concentrations.
Different methods of testing for nitrites have been developed on a laboratory scale with positive results in both drinking water and different foods.
The main sectors of application are pollution and environmental impact and the agro-food sector, since this invention may represent a great progress in the detection of nitrites in water and food.
The research group is looking for companies interested in acquiring this technology for commercial exploitation through patent licensing agreements.
This technology is protected by patent application. This technology is protected by national and international patent applications.
• Title of the patent: “Formilación de indolizinas y detección de nitritos”
• Application number: P202030552 PCT/ES2021/070173
• Date of application: 09/06/2020 10/03/2021
Pollution and Environmental Impact
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959





