Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Institute of Organic Synthesis of the University of Alicante has developed a simple and economical synthetic route to obtain a new family of molecules (zwitterions of saturated, monounsaturated, polyunsaturated 2-phosphocoline carboxylic acids and their acid derivatives) that are characterized by their activity against different types of carcinogenic cells.
These compounds have been synthesized at laboratory scale and studies of antitumour activity have been carried out by measuring in vitro the IC50 index of different human tumour cell lines with excellent results.
Companies in the pharmaceutical industry interested in acquiring this technology for commercial exploitation are sought.
Nowadays, it is well known that cancer is, behind cardiovascular diseases, the second leading cause of death in the world, causing 14 million deaths in 2012. In order to treat this disease, anti-cancer agents are sought that are capable of selectively attacking cancer cells, leaving healthy cells unaltered. In this sense, lipid derivatives of different nature have demonstrated their antitumor effectiveness, among other applications.
Over the last few years, phospholipid derivatives have been developed with different applications: transdermal and transmembrane, anti-cancer or for the treatment or prevention of arteriosclerosis and other related disorders, as well as inflammatory processes, autoimmune diseases and proliferative disorders, among others.
Although many compounds have been obtained showing a high metabolic stability and anti-cancer effects, these have drawbacks such as low solubility in water or high toxicity. Other compounds with identical properties presented the problem that, for their synthesis, hard reaction conditions were used (temperatures between 110º and 120ºC), which do not allow the synthesis of sensitive compounds.
Therefore, it is necessary to search new chemical formulations that are economical and synthetically simple to carry out and highly active against different lines of tumour cells.
The Institute of Organic Synthesis of the University of Alicante has developed a procedure to synthesize a new family of molecules suitable for use in the prevention or treatment of cancer due to their cytotoxic properties.
The present invention refers to the zwitterion of saturated, monounsaturated, polyunsaturated and carboxylic 2-phosphocolinic acids and their pharmaceutically acceptable derivatives, with a structure described by the general formula (1):
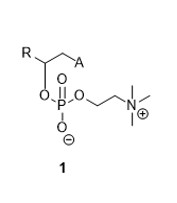
Where:
• A = saturated, monounsaturated or polyunsaturated carbonate chain, with any configuration in the double bonds C=C, mono or polyhydroxylated in different positions, or non-hydroxylated.
• R = An atom or group of atoms weighing between 1 and 200 Dal, and their pharmaceutically stable derivatives (e.g. salts, hydrates or polymorphs).
The novel synthesis procedure for this family of compounds consists of two reaction steps (Schemes 1 and 2) under mild reaction conditions. The corresponding 2-hydroxy derived, obained from saturated, monounsaturated, polyunsaturated acid or its derivatives is transformed into the Zwitterion of saturated, monounsaturated or polyunsaturated 2-phosphocolinic carboxylic acid or one of its derivatives:
(i) Generation of an intermediate compound of general formula (2) by the addition of 2-bromoethyl dichlorophosphate of formula (3), or its chlorinated or iodinated analogue, on the hydroxyl group in alpha position to the carbonyl group, of a compound of formula (4) in the presence of a nonpolar solvent at ambient temperature.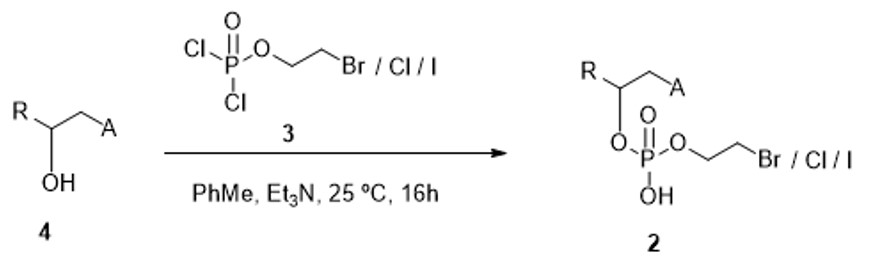
Scheme 1. Generation of the intermediate compound of general formula (2). Both in formula (2) and in (4) A and R have the meanings given for the compound formula (1).
(ii) Replacement of the bromine atom (chlorine or iodine) of the intermediate compound of formula (2) by a trimethylamino group and formation of the corresponding betaine is performed. For this, the intermediate of the previous step (2) is reacted with trimethylamine dissolved in an alcohol, in mild conditions between 0ºC and 60ºC for 1-5 days and subsequent treatment with a mild inorganic base.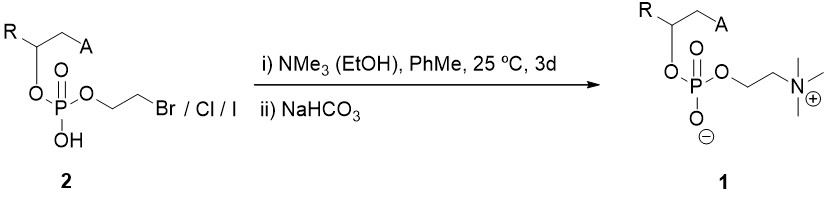
Scheme 2. Substitution reaction to form the corresponding betaine of the compound with general formula (1).
It has been observed that the general formula molecule (1) shows in vitro activity against different types of carcinogenic cells, for example against tumour cell lines: lung (non-small cell), pancreas, kidney, central nervous system (CNS), prostate, colon, breast, melanoma, ovary, acute lymphoblastic leukaemia, acute promyelocytic leukaemia, chronic myelogenous leukaemia, myeloma, large cell immunoblastic lymphoma, Burkitt lymphoma, non-Hodgkin's B-cell lymphoma, chronic lymphocytic leukaemia, mantle cell lymphoma, multiple myeloma and acute T-cell leukaemia.
The main advantages of this technology are the following:
• The synthesis of these compounds takes place under mild reaction conditions.
• The reaction conditions used improve the synthetic operation on a larger scale and the execution times are shorter.
• The products obtained do not present neither problems of solubility in water nor toxicity.
• The series of molecules obtained present preventive or curative activity for a great variety of carcinogenic cell lines (IC50 up to 7.06±1.45 µM).
• The synthesized zwitterions also present less degradation in the biological environment, which implies a greater therapeutic effect, as well as greater specificity towards the lipidic bi-layer of the cells and its action on them.
MAIN INNOVATIVE ASPECTS OF THE TECHNOLOGY
The main innovative aspect of this technology is the invention of a new family of zwitterionic molecules of saturated, monounsaturated, polyunsaturated acids-phosphocholine and their acid derivatives with the general structure (1) - whose characteristics confer the molecule a preventive or curative activity in the micromolar range for a wide variety of carcinogenic cell lines.
In addition, these new chemical formulations have been achieved using a simple and inexpensive synthetic route.
A series of compounds with the general formula (1) have been synthesised on a laboratory scale.
In a particularly preferential feature of the invention, A is a fragment of fifteen carbon atoms containing a double C=C bond between the C9-C10 carbons with Z configuration and the group R represents an ethyl ester COOEt (molecule MCH-811). Antitumour activity has been studied by measuring in vitro the IC50 index of different human tumour cell lines (pancreas, lymphoma, lung, kidney, etc.).
The current invention is framed as much in the field of the pharmaceutical chemistry as in the medicine area, with more specific emphasis to those compounds useful as cytotoxic agents.
Formula compounds (1) and their pharmaceutically acceptable derivatives such as salts, hydrates or polymorphs, can be used as active species against different types of carcinogenic cells, preferably in the prevention and/or treatment of cancers such as those mentioned in the technical description.
The research group is looking for companies interested in acquiring this technology for commercial exploitation through:
• Patent licensing agreements.
• Technical cooperation (R&D projects) to develop new molecules, new applications, carry out industrial scaling, adaptation to the specific needs of companies, etc.
• Etc.
This technology is protected by patent application.
• Title of the patent: "Zwitterionic compounds of carboxylic 2-phosphocolinic acids and their use as cytotoxic agents".
• Application number: P201830802
• Application date: 02/08/2018
Medicine and Health
Chemical Technology
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




