Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Adhesion and Adhesives Laboratory of the University of Alicante has developed a new polymeric foaming material for in-situ filling and sealing of internal irregular different shaped human cavities, intended for patients suffering chronic pleural cavities and in-field injuries caused by bullets or accidental event leaving open blood vessels.
The new foam is composed of two separate liquid components that can be mixed in a two-body syringe in such a way that the foaming time can be modulated for allowing the foam formation at the end of the syringe needle. The new polymeric foam can self-expanded and self-modelated for avoiding complications in open internal cavities caused by infections, blooding, fistulae, dyspnea or sepsis. The new foam is easily applicable, safe for biological tissues, and its use avoid the use of the current aggressive treatments in pleural cavities. It is looking for companies interested in acquiring this technology for its commercial exploitation.
The pleural cavity contains a minimal amount of liquid for maintaining the negative pressure needed for an adequate pulmonary function. Several pathologies of the pleural cavity produce air or biological liquids (pus, blood, pleural openings, etc.) that are commonly solved by draining. However, some patologies cause chronification leading to severe clinical complications such as infections, sepsis, fistulae, pain, hemorrhages or disneas.
The current treatments for draining the pleural cavities are mainly decortications, toracoplasties, toracotomies, pedicular inserts, thoracic openings, and filling with different synthetic materials.
The most commonly used synthetic materials are: air, polymethylmethacrylate hollow spheres, breast or testicular implants, and expanded prostheses filled with sterile liquids. However, none of these materials is sufficiently efficient as they are aggressive and may cause severe clinical complications such as infections of fistulae, and this cause an important deterioration of the quality of life of the patients.
One more recent treatment is the Vacuum-assisted closure (VAC) that consists in the disinfection of the pleural cavity followed by application of one foam acting as a vacuum pump that favour healing and dreaning. Another more recent synthetic materials used for filling the cronic pleural cavity are copolymers of lactic acid and caprolactone but they are not self-expandable, they show insufficient mechanical properties and they cannot be injected throughout small cavities. There are some polyurethane foams for covering silicone breast and testicular prostheses but they have not been used for filling pleural cavities yet.
Currently, several polyurethane foams intended for biomedical applications have been developed following two different approaches :
1) Polyurethane foams containing different functional compounds for imparting self-adhesion properties. These foams are effective against gram-positive and gram-negative bacteria, and they are biocompatible.
2) Mixing of thermoplastic polyurethanes and biodegradable polyesters derived from polilactic acid able to produce closed-cell foams with good contraction and biocompatibility. Although these foams are currently used for drug delivery and medical patches, they have not been used for filling pleural cavities yet.
The Adhesion and Adhesives Laboratory of the University of Alicante has developed a new polymeric foaming material for in-situ filling and sealing of internal irregular different shaped human cavities, intended for patients suffering chronic pleural cavities and in-field injuries caused by bullets or accidental event leaving open blood vessels.
The new polymeric foams show a solid and a gas phase dispersed into the polymeric matrix, forming discrete or interconnected cells. The formation of in-situ the polyurethane foams is reached by mixing two liquid components that can be injected by means of a double-container syringe. The two liquids components may have different compositions :
• Component 1: it may contain water, polyols, foaming agents, catalysts, surfactants, other polymers, plasticizers, inorganic and/or organic fillers, processing aids, rheological additives, radiological tracers and antibiotics, among other.
• Component 2 : it contain the isocyanate of isocyanates mixture.
The two components are liquid at room temperature and they can be mixed in few seconds before injecting in a syringe for dosing in a liquid form in the internal human cavity (pleural cavity, blooding vessel, injury in the field), in such a way that the foaming can be controlled for being fast of slow (less than one minute in any case). The composition is designed for starting the foaming during the application of the two liquid components avoiding, that the liquids can be in contact with the tissues surrounding the cavity.
The component 1 can be homogenized by mechanical stirring in less than one minute.
As the component 2 is a liquid, it can be easily manipulated.
The two liquid components can be mixed by mechanical stirring in less than 20 seconds. By changing the ratio of the two components, the foaming process can be controlled for adapting to the specific cavity and to different geometries, shapes and size of the cavities.



Some polyurethane foams with different extent of foaming produced by using different formulations.
MAIN ADVANTAGES OF THE TECHNOLOGY
The new polyurethane foams show the following particularities:
• They are easy to apply, even throughout very small orifices.
• They are self-expandable and self-modelled as by controlling the time after mixing the two components and in the presence of moisture, they expand spontaneously, filing the cavity completely.
• They show null adhesion to the surrounding tissues, and their surfaces are impermeable.
• They have very low density (i.e. low weight).
• The foam can be extracted easily from the internal cavity.
• They are biocompatible and show low risk of tocixity, carcinogenesis and local clinical complications.
• They can be dosed into open or closed cavities.
• They can be applied in the presence of blood or biological liquids.
• The resulting foams do not deteriorate with time and are stable.
The new polyurethane foams can be formulated for rending flexible, semi-rigid or rigid foams, and in all cases, an homogeneous size and cell distribution is obtained, and all them show adequate mechanical resistance.
INNOVATIVE ASPECTS
The new polyurethane foam is intended for filling internal human or animal cavities having different shapes and sizes, showing the following particularities:
• Lack of toxicity, hypersensibility, carcinogenesis, post-surgical and complications.
• It does not cause infections.
• It does not affect the healing and fistulae formation is not favoured.
• The full cavity can be completely filled irrespective of its size and shape.
• The foam has a poor adhesion to the surrounded tissues.
• The foam is light (i.e. low weight), and it can be easily removed, if necessary.
• The foam does not interfere the heart or diaphragma movements.
• The foam is impermeable to biological fluids, water and blood.
• The foam has hemostatic effect.
• The foam allows the sealing of parenchima or bronquial fistulae.
•The foam can be easily applied and dosed into the internal cavities.
• The foam is stable over time and does not deteriorate in contact with biological fluids.
The technology has been developed at laboratory scale. A proof of concept has been tested for determining the viability of the new polyurethane foam in post-neumonectomy cavity in 20 Sprague Dawley rats and 6 rabbits.
Neumonectomy was carried out in each animal and the volumens of the cavities were determined. The two components of the new polyurethane foams were injected by means of a syringe into 1/3 of the cavity volume. Then, the thoracotomy was closed. Monitoring of the polyurethane foam was carried out by using X-ray equipment. After 3 months, the animals were sacrificed and the polyurethane foams were extracted and analyzed.
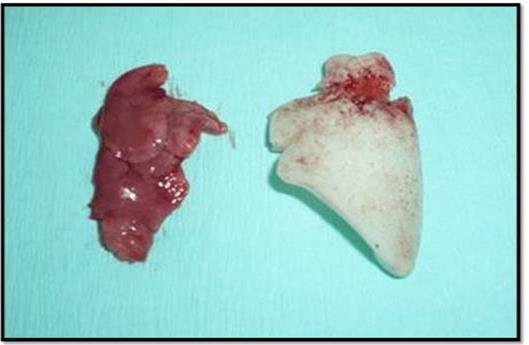
Photos of left lung of Sprague Dawley rat. The excellent adaptation of the polyurethane foam to the pleural cavity can be noticed.
The analysis of the extracted polyurethane foams show the following evidences:• The foams filled completely the cavity and they were adapted perfectly to the pleural cavity of the animals.
• The mechanical integrity of the foams was maintained and it was excellent.
• Ipsilateral displacement was not observed.
It is looking for companies interested in acquiring this invention for commercial exploitation through the following ways:
• License agreement of the patent.
• In search for financial opportunities to develop new applications, adapt them to specific needs of the company, etc.
• Agreements for technology and knowledge transference.
• Technical reports and scientific assessment.
• Offer specific training depending on the companies needs.
• Standardization services, calibration, national and international technical rules, etc.
• Offer technological support on those technologies that require high preparation or sophisticated instruments that are not in the companies grasp.
• Staff exchange for specific periods of time (to learn specific techniques).
Rent the internal equipment to clients that wish to continue their own tests (the infrastructure of the Department of Inorganic Chemistry – Adhesion and Adhesives Laboratory - or the Technical Services of Research of the University of Alicante (SSTTI)).
The present invention is protected by granted patent:
• Title: “Uso de espumas poliméricas autoexpansibles para el relleno de cavidades pleurales persistentes”.
• Application number: P201531167.
• Application date: 5th August 2015.
Medicine and Health
Chemical Technology
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959





