Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Molecular Nanotechnology Laboratory of the University of Alicante has developed a new hybrid zeolitic material characterized by the fact that the same solid contains, at least, two different zeolites. With this innovative solution, new more efficient and sustainable "ad hoc" catalysts can be developed for different industrial processes, including: chemical (oil cracking), pharmaceutical (increasing the performance of synthesis processes by more than six times), reuse of plastics, waste valorization, etc.
This novel technology improves the performance of chemical reactions, it increases catalytic activity in the transformation of bulky molecules and it allows significant energy savings. The technology has been developed at laboratory level, and it has been successfully validated in different chemical processes with different molecules.
It is looking for companies interested in acquiring this technology for its commercial exploitation through patent license agreements.

Zeolites are minerals formed by a crystalline structure containing aluminium and silicon atoms. They can be natural or synthetic, and they are characterized by their ability to hydrate and dehydrate in a reversible manner.
Zeolites have a microporous structure (they have channels and cavities of molecular dimensions), and together with their strong acidic character and excellent physical and hydrothermal stability, make them very suitable for a multitude of applications in the chemical industry, such as: molecular sieves, adsorbents, detergent additives and, especially, as catalysts.
Despite the fundamental role of zeolites in catalytic and adsorption processes, the intrinsic narrow microporosity of these materials limits their use for large molecules for molecular orientation or diffusion reasons.
In this sense, bulky molecules only have access to active sites located near the entrance to the zeolite pores, or on the outer surface of the zeolite, which only represents 5% of the total zeolite active surface area.
To solve this important limitation, two fundamental strategies have been developed:
a) Generate mesopores (diameters between 2-50 nm) in the zeolite structure, initially microporous (with diameters smaller than 2 nm).
b) Reducing the size of the zeolite to the nanometer scale.
The application of both strategies has resulted in six main types of zeolitic materials:
• Nanozeolites.
• Zeolites with intracrystalline mesopores.
• Zeolites supported in mesoporous matrix.
• Ordered mesoporous materials assembled from nanozeolites.
• 2D zeolites.
• Zeolite nanosheets.
Currently, there are three procedures to synthesize zeolites with controlled porosity:
1) Generate mesopores through treatments with: water vapor, acids, bases or other chemicals on zeolite crystals. However, this method causes a significant deterioration of the zeolitic structure.
2) From precursors including zeolite seeds. With this method, mesoporous solids with a low concentration of zeolite fragments are obtained.
In view of the limitations described above, and given the wide application of zeolites at industrial level in a multitude of processes, it is necessary to design and develop new zeolitic materials of hybrid type, that is to say, that combine in a single material the best properties of several of the known zeolites to optimize and improve their industrial performance.
In order to solve the described problems, a new hybrid zeolitic material has been developed whose structure is formed by a combination of different zeolitic structural units belonging to, at least, two different zeolites. The zeolitic structural units are finite or infinite building units (in the form of a chain or layer) that make up the zeolite structures.
The zeolitic structural units that make up the hybrid material belong to, at least, two of the following types of zeolites:
• Faujasite (FAU).
• Beta (BEA).
• Invertedordenite (MFI).
• Chabazite (CHA).
• Mordenite (MOR).
• Zeolite type L (LTL).
• Ferrierite (FER).
• Linde type A (LTA).
The obtained hybrid zeolitic material is partially or substantially amorphous, i.e., at least 90% of its structure is composed of the unordered three-dimensional repetition of its unit cell.
This novel material has a significant mesoporous structure, i.e. it has a porosity between 0.05-1.5 cm3/g (see Figure 1) and, in addition to silicon and aluminium atoms, it can also contain other types of elements (e.g. gallium, iron, germanium and/or phosphorus atoms).
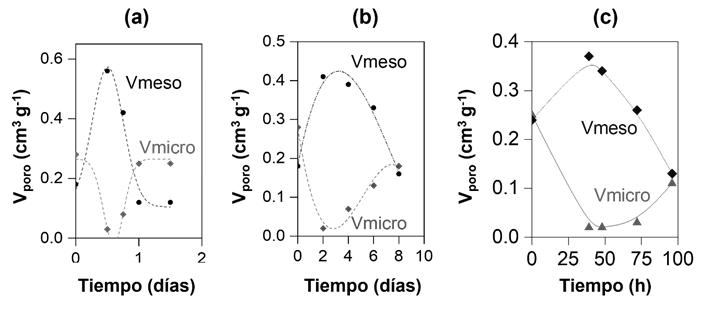
Figure 1: time evolution of the crystallinity (mesopore and micropore volume) of the hybrid material obtained by partial interzeolitic transformation at different hydrothermal treatment time periods and in the presence of: (a) a structure directing agent; (b) a structure directing agent and a cationic surfactant; and (c) a structure directing agent with surfactant properties.
Additionally, the surface of the hybrid zeolitic material can be functionalized by hydrophilic or hydrophobic treatment, or by reaction with alkoxylanes ending in amine, phosphine or carbonyl functional groups. In this way, the material is provided with a new chemical functionality, either by this functionalization, or because through the functional groups incorporated on the surface, other molecules, nanoparticles or metals are anchored, generating new physicochemical properties, thus increasing its industrial applications.
To obtain these innovative hybrid materials, the following synthesis strategies have been proposed:
A. Partial interzeolitic transformation:
It consists of converting, partially, the structural units of one type of zeolite into structural units of a different zeolite. For this purpose, the following steps are carried out:
1) Mix, at least, one zeolite with a structure directing agent in a basic solution. Additionally, a cationic surfactant can be introduced to control the size and volume of the pores in the resulting hybrid zeolitic material, although the structure directing agent can also has surfactant properties.
2) Subject the above mixture to a certain temperature (below 220°C) for a specific period of time.
3) Finally, the zeolitic hybrid material obtained can be filtered, washed, dried and calcined. It could also be mixed with a binder (e.g. clay, alumina and/or silica) by extrusion or flash drying.
With this method, it is achieved that, at least, 90% of the resulting hybrid zeolitic material comprises zeolitic structural units different from those of the original starting material (see Figure 2).
B. Partial dissolution of several different zeolites and subsequent precipitation:
This method comprises the following steps:
1) Obtaining a set of zeolitic structural units by partial and independent dissolution of, at least, two different types of zeolites in a basic aqueous solution at a certain temperature for a specific time.
2) Mixing the zeolitic structural units obtained in the previous step.
3) Adjust the pH to a certain value.
4) Add a precipitating agent.
In B method it may be necessary to separate the structural units obtained (either from partially dissolved zeolite residues or from zeolite fragments that may have formed) by centrifugation, filtration or decantation, among others.
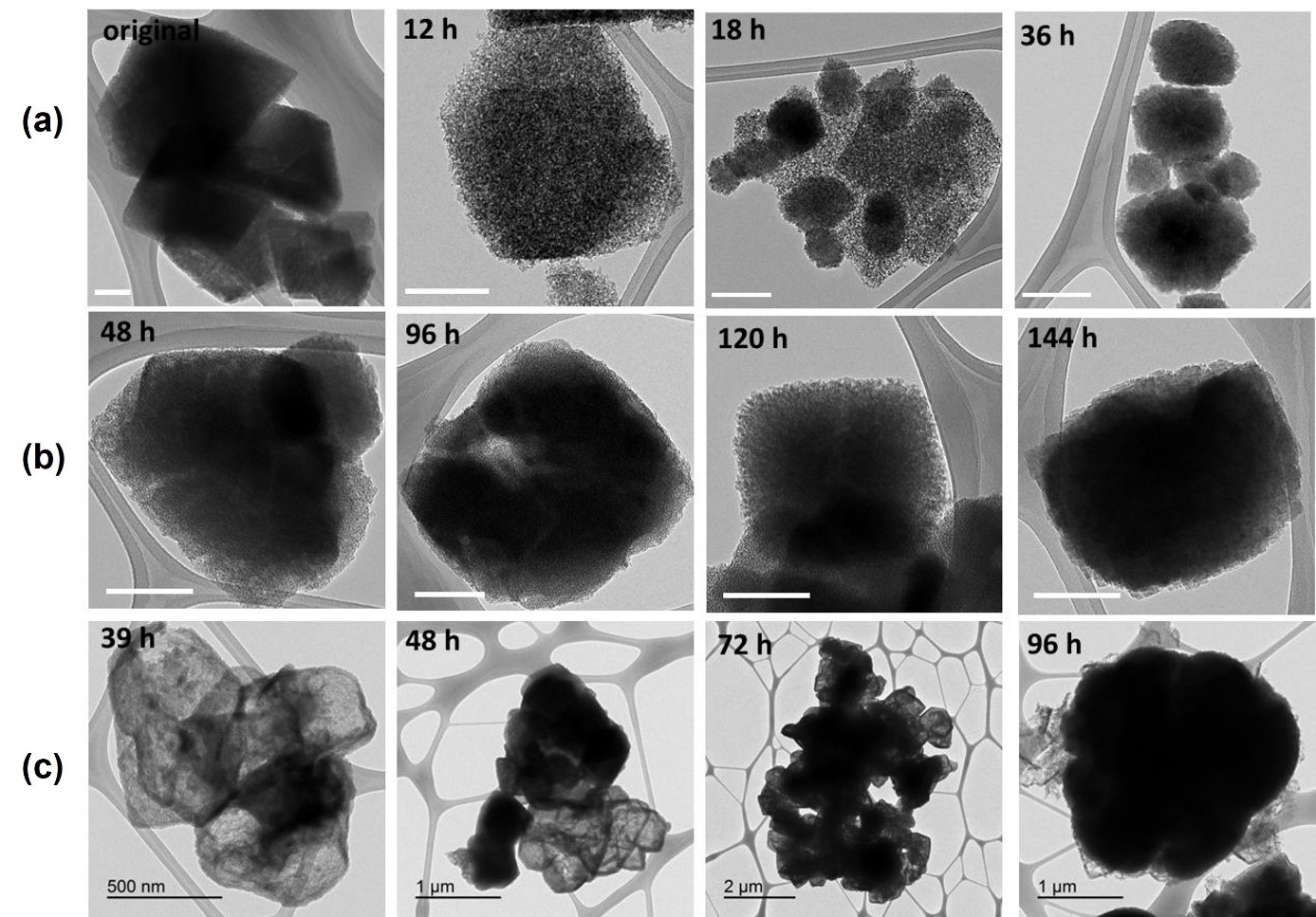
Figure 2: Transmission Electron Microscopy (TEM) images of the hybrid zeolitic material obtained by partial interzeolitic transformation at different hydrothermal treatment times.
ADVANTAGES OF THE TECHNOLOGY
The design and development of new "on demand" hybrid zeolitic materials has the following advantages:
1) The inclusion of mesopores in the zeolitic structure significantly shortens the diffusion path of reagents and products, which it reduces the contact time and it improves the overall performance of the material.
2) Enhanced catalytic activity in the transformation of bulky molecules.
3) Improved hydrothermal stability compared to similar purely amorphous materials.
4) Very precise control of the size and volume of the pores in the resulting hybrid material, as well as in the relative amount of the fragments of the different zeolites.
5) Improved technical performance: physicochemical properties (stability, acidity, confinement, etc.) can be adjusted specifically to the industrial application of interest.
6) The addition in the structure of other elements than silicon and aluminium allows improving some of its properties, such as acidity, activity or catalytic selectivity, as well as providing the hybrid material with new functionalities, such as: redox properties, different hydrophilicity, reactivity and affinity for different molecules.
7) Higher yields in the production of fossil fuels and in the synthesis of pharmaceutical compounds (up to more than six times).
8) The preparation procedure of these materials is very simple.
9) The synthesis method used is sustainable and environmentally friendly.
10) Great versatility: the physicochemical characteristics of the synthesized catalyst can be modified with great precision to suit the needs of each process.
11) The synthesis process saves energy and natural resources compared to current conventional zeolite synthesis methods: for example, it is possible to significantly reduce the temperature required to degrade different types of plastics, which it means significant savings in energy and CO2 emissions.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
A new family of catalysts with "on demand" properties has been developed whose main innovation lies in the fact that they can be designed with optimized physicochemical features for different industrial processes.
A novel material has been synthesized that combines the best characteristics of several zeolites. For this purpose, an ingenious and simple procedure has been used to transform zeolites into other more stable. Through precisely controlling this conversion, the characteristics of several zeolites have been achieved in a single material, which represents a very promising advance in the field of catalysis.
In this sense, synthesizing catalysts that combine the properties of several zeolites opens up countless opportunities in sectors such as pharmaceuticals, chemicals or the reuse of plastics (see Images 1 and 2).


Image 1 and 2: vials with the new catalysts designed at the UA, and sample of plastic transformed into hydrocarbons.
The new hybrid zeolitic materials have been synthesized at laboratory scale and they have been successfully validated in different chemical processes with several molecules. The technology is at a stage of maturity corresponding to Technology Readiness Level (TRL) = 3.
The great potential of these new materials in different applications in the chemical, pharmaceutical and energy industries has been demonstrated, as well as their huge economic and sustainability advantages (see Figure 3).
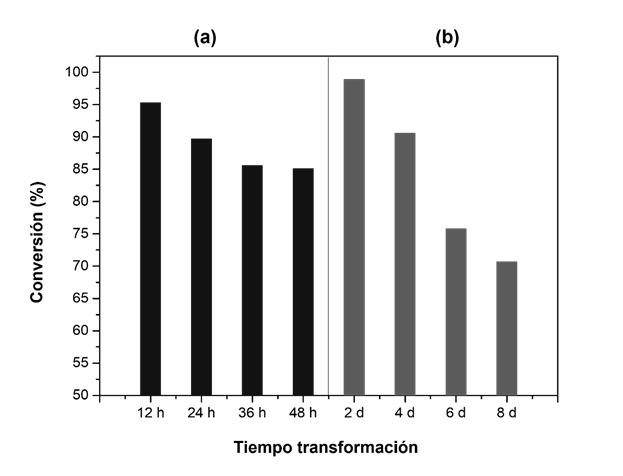
Figure 3: catalytic activity of the hybrid zeolitic material obtained under two different ways, and evaluated from the Friedel-Crafts alkylation reaction between indole and diphenylmethanol.
This technology is part of the field of materials chemistry, and it finds its main applications in industry:
• Chemical.
• Pharmaceutical.
• Energy.
• Reuse of plastics.
• Waste recovery.
• Elimination of pollutants.
• Ionic exchange.
• Adsorption:
o Drying.
o Purification.
o Separation.
• Catalysis:
o Catalytic cracking (oil refining).
o Hydrocracking.
o Alkylation.
o Acylation.
o Isomerization.
o Oligomerization.
o Hydrotreating.
o Biomass transformation.
Companies interested in acquiring this technology for commercial exploitation through patent licensing agreements are sought.
Company profile sought:
• Chemical, pharmaceutical, energy, catalysis, etc.
The present invention is protected through patent application:
• Patent title: "Material zeolítico híbrido, métodos de obtención y usos asociados".
• Application number: P202230159.
• Application date: 28th February 2022.
Pharmacology, Cosmetics and Ophthalmology
Materials and Nanotechnology
Chemical Technology
Transport and Automotive

Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




