Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Plant Proteomics and Functional Genomics research group at the University of Alicante has designed and manufactured a bubble column-type bioreactor to carry out the culture of cell a cell suspension of any type of plant in aseptic conditions. Its novel design allows to recover the culture medium, to replace it with another medium, and to reuse the remaining biomass for a next culture operation. This novel bioreactor is characterized by its low cost and because it allows a homogenous and efficient aeration, and the correct agitation of the culture. We look for companies interested in licensing this technology for commercial exploitation.
Bioreactors are widely used for growing microorganisms (bacteria, fungi, etc.), both on a laboratory and industrial scale. Their design, usually stirred tank driven by mechanical devices, is not always suitable for plant cell suspension culture. These are very sensitive to the shear stress, display a low oxygen demand, a low growth rate, and use to form large aggregates.
In order to design an appropriate bioreactor for a particular bioprocess, it is necessary to know both its cell growth pattern, its metabolism as well as other factors. In addition, it is necessary to optimize and control the bioreactor operating parameters (concentration of dissolved oxygen, pH, temperature, mixing, nutrient supply, etc.) to support the functions of cell survival and production of metabolites of interest.
The existing bioreactors for plant cell culture, usually bubble column- or airlift-type driven by pneumatic turbulence, display the need of a homogenous and constant culture mixing as the main problem to cope with.
Currently, there exists a trend towards the use of disposable plastics as biorreactor building materials, however, the high costs in consumables that go with it are only justified if the obtained products have high market value (for example, antibodies, vaccines, therapeutic proteins, etc. obtained through mammalian or insect cell cultures).
Plant cells are grown to produce both biomass and natural compounds. In both cases, the replacement of the culture medium may be necessary, which is an important problem as the manipulation of the culture itself implies a risk of contamination and thus of culture complete spill.
The present invention solves the technical problems described above as its novel design allows the optimal plant cell culture, thus overcoming the limitations in both biomass and bioactive compounds production in which the exchange of the culture medium is required.
This invention relates to a novel bioreactor that allows for the growth of a plant cell suspension under aseptic conditions in a liquid medium whose composition fulfills the nutritional and physiological requirements of the cells under appropriate physicochemical conditions.
The bioreactor is composed of the following three parts to be assembled and sterilized (Figure 5):
1) Body (Figure 1): it is a hollow container of one piece, of circular section, with straight walls and curved bottom. The body comprises a spherical and a double-wall cylindrical part (with inlet and outlet piped holes) through which a thermostated liquid can flow to regulate and maintain constant the culture temperature. Both parts are internally separated through a porous wall (glass or steel) whose mesh size allows the constant and uniform flow of fluids between them, but not of particles larger than the pore size. The upper part of the body is finished with a horizontal flange that allows it to rest on the bracket, and thus the cap rest on the body to get a hermetic seal. The spherical part has, at least, two piped holes to connect fluid inlet / outlet: one connected to a sterile (oil-free) air line with adjustable flow rate, and another for liquid or gas flow, thereby achieving a homogeneous supply of air to the plant cell culture, and an efficient mass transfer. The body can be transparent or opaque, and glass or steel.
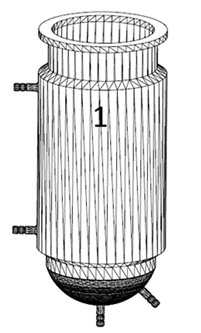
Figure 1: Design of the bioreactor body.
2) Cap (Figure 2): it is a solid cylindrical stainless steel part with several holes to be able to screw it to the support and to attach other accessories (plugs, aseptic sampling tubes, filling tubes, sensors etc.). It is used to close the open top of the body. It has short ports for connecting flexible pipes for the addition of fluids and for the gas inlet / outlet. On its inner side, there is a peripheral groove to be able to couple a rubber or silicone sealing ring that allows the bioreactor to be a hermetic seal.
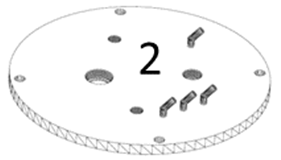
Figure 2: Design of the bioreactor cap.
3) Bracket (Figure 3) : it is a piece of stainless steel with, at least, four tubes in equidistant parallel arrangement, welded at the end of ground support to a tube that forms an incomplete circumference, and on the other side, to a solid ring which has several holes that match with those of the cap so that they can be joined by screws running through both pieces. The ring has welded handles to facilitate the transport of the bioreactor, and three screwed on pieces of flexible material where the body can rest to avoid fracture of the glass because of the strains. The bracket allows the attachment of the body and the cover.
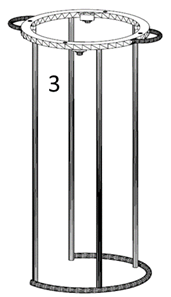
Figure 3: Design of the bioreactor support.
Additionally, the bioreactor can be connected to reservoirs (Figure 4) for liquid exchange. These reservoirs are connected through autoclavable silicone hoses to the inlets and outlets of the bioreactor.
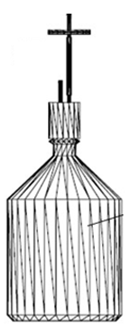
Figure 4: Reservoir for the exchange of liquids with the bioreactor.
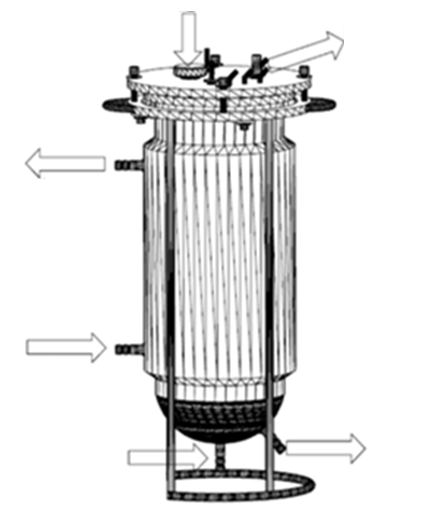
Figure 5: Assembled bioreactor parts.
The operation of the bioreactor can be summarized as:
1. Preparatory sterilization operations are carried out.
2. The sterilized assembled bioreactor is filled or emptied: the aseptic emptying / filling cycle can be repeated as many times as desired (Figure 6).
3. The next step is aeration and pneumatic mixing of the filled bioreactor: air flow is conducted through the porous base, resulting in a fine sparging effect in which the air dispersed as tiny bubbles rises freely producing an effective aeration and stirring effect.
4. The bioreactor is inoculated in an aseptic environment with a plant cell suspension. It derives from callus of dedifferentiated cells from plant tissue cultured in vitro.
5. Growth of plant cells in "batch" mode from the inoculum of the previous cell suspension. The aeration favours homogeneity and growth of the culture until the stationary phase of growth is reached, or until the desired amount of biomass is obtained.
6. Recovery of the culture by disassembling the cap and transferring it to another container.

Figure 6: Emptying operation of the bioreactor.
In addition, it is possible to culture plant cells in "fed batch" mode from a pre-culture in situ. After batch cultivation, the nutrient-depleted medium is displaced to an empty reservoir. The biomass remains in the bioreactor, which is then filled with fresh medium rich in nutrients to continue the cell growth as described in point 5 above for the "batch" mode. This operation can be repeated as many times as desired. When the last cycle ends, the culture is recovered as described in point 6 above.
Some examples of plant cell culture are described below using the bioreactor described herein (7-liter prototype):
Example 1: Culture of a Vitis vinifera cell suspension in batch mode --> the culture temperature was held constant at 24°C and aeration was adjusted between 0.3 and 0.6 l.l-1.min-1. The culture reached the stationary phase after 28 days, achieving a 7-fold increase of the initial biomass.
Example 2: Extracellular production of the natural compound trans-resveratrol in "batch" and "fed batch" modes by a Vitis vinifera cell suspension at 24°C and aeration at 0.43 l.l-1.min-1. --> an extracellular concentration of trans-resveratrol higher than 3 g/L was achieved, being able to re-use the biomass obtained up to 3 production cycles without loss in the yield. The average length of each cycle was 4 days, with an average resveratrol production of 15 g/cycle.
Example 3: Extracellular production of trans-resveratrol in "fed batch" mode coupled to a "batch" culture of a Vitis vinifera cell suspension --> an 8-day culture cycle was carried out at 24°C and aeration between 0.47 and 0.54 l.l-1.min-1, followed by 3 production cycles at 24°C and aeration at 0.58 l.l-1.min-1, with an average duration of 5 days/cycle, and with an average resveratrol production of 12 g/cycle.
INNOVATIVE ASPECTS AND ADVANTAGES OF THE TECHNOLOGY
This novel bioreactor has the following advantages over today's commercially available stirred tank or airlift bioreactors of similar size:
1) It is a low cost bioreactor.
2) It allows homogenous and efficient pneumatic aeration and agitation, even at high cell densities.
3) Its design allows for working in a permanent aseptic environment throughout the process.
4) Its design allows a R3:
• Recover the culture medium.
• Replace the culture medium.
• Re-use the biomass for the next culture operation.
6) The design is adapted to the growing needs of any type of plant cell culture in suspension.
7) Reduction of operational cost versus single use (disposable) models.
9) The design is adapted to the needs of plant cells culture in suspension.
A 7-liter prototype (Figure 7) has been designed and built for the laboratory scale tests that have been carried out. In its construction, re-usable materials (glass and metal) have been used. The following table shows the characteristics of oxygen transfer and mixing time at different aeration volumes.
Oxygen transfer rates and liquid mixing time at different aeration volumes:

Figure 7: 7-liter prototype.
The Oxygen Radical Absorbance Capacity (ORAC) analysis of trans-resveratrol obtained from Vitis vinifera cell cultures using this prototype has a relative ORAC value of 4.40, while commercial trans-resveratrol of purity > 98% has an ORAC value of 4.68, so the antioxidant power of the product obtained is 94% compared to the pure product.
After carrying out a cost analysis, it has been concluded that the commercial production of trans-resveratrol using Vitis vinifera cell cultures with this 7-liter prototype, is around 500 €/kg.
The present invention is framed in the field of Biotechnology. In particular, it refers to a bioreactor to carry out, under aseptic conditions, plant cells culture in suspension with the aim of obtaining biomass or metabolites or both with commercial interest for the following sectors:
• Cosmetic.
• Pharmaceutical.
• Cleaning and personal care.
• Nutraceuticals.
• Food.
• Agricultural.
We look for companies interested in licensing this invention for commercial exploitation through:
• License agreement.
• Technology and knowledge transfer agreements.
• Provide technological support in those techniques that require high training or sophisticated instruments that are not available to the requesting company.
The present invention is protected through patent granted with prior examination:
• Title: "Biorreactor tipo columna de burbujeo para cultivo de células vegetales en suspensión".
• Application number: P201730479.
• Application date: 30th March 2017.
Biology
Molecular Biology and Biotechnology
Pharmacology, Cosmetics and Ophthalmology
Engineering, Robotics and Automation
Medicine and Health
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959





