Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
A multidisciplinary group of researchers from the University of Alicante has developed a new system for the formation of artificial marine reefs and underwater structures with porous calcareous coating using electric current.
The system is characterised by the fact that it starts from a lightweight metal base of any geometric shape –with the possibility of assembly– and the final structure can be easily transported and placed, or fabricated in-situ. During the processing stage, substances are released into the environment that favour the development of phytoplankton and are harmless to the habitat, thus establishing a greater diversity of marine species on the structure itself.
This device allows the restoration of marine ecosystems, the purification of marine waters in aquaculture farms, as well as the sustainable exploitation of marine leisure (recreational diving).
Companies interested in acquiring this technology for commercial exploitation through licensing agreements are being sought.

In order to alleviate this progressive degradation, different technologies have been developed to form artificial marine reefs. Some of these technologies and their main drawbacks are presented below:
Artificial reefs with formation of calcareous deposits by electrolysis:
• There are various metallic elements coated with calcareous deposit:
o They do not have the possibility to form stackable elements in the form of mesh cylinders, which are lighter and more effective for the attachment of filter-feeding organisms.
o They also do not allow for a high interaction of marine species in the openings of the mesh size, and have high transport costs.
o They do not consider the geometry of the anodes, nor their relationship to the deposit formed.
o Nor do they consider the possibility of avoiding or minimising the effect of acidification by using iron anodes, or the benefit that this type of anode has for the development of phytoplankton.
o They do not use external current in the electrolysis of seawater as an accelerator method of the procedure.
o They do not have the possibility of using the same material for the anode and cathode.
o The anode material does not add value to the process and may generate environmentally harmful substances (heavy metal ions).
Artificial reefs without the use of electrolysis:
• Concrete blocks with steel reinforcement and the possibility of bonding between them: the high pH of the solution that exists in the network of pores of the concrete makes it necessary to incorporate iron sulphate to counteract this alkalinity. In addition, they are heavy (which complicates their transport, anchoring and placement), they are expensive, and the colonisation times are very long, as they are not covered by calcareous films (similar to those excreted by corals).
• Concrete modules with silica fume and glass fibre reinforcement rods: cannot be manufactured in-situ. They are heavy and complex to transport and handle.
• Tyres used on skeletons made of various materials: these are unnatural elements in the marine environment, and are not the most suitable option for the attachment and growth of marine organisms.
• Various plastic materials such as polyvinyl chloride (PVC): these materials do not have a suitable surface for the attachment of marine organisms, and their degradation causes a gradual contamination of the marine environment by microplastics.
Therefore, there is a need to develop a system that allows the formation of marine reefs in an environmentally friendly way (both for the habitat and the species themselves).
In order to reduce the problems described above, a simple method has been developed to form marine reefs and underwater structures with a stony coating of calcium carbonate (CaCO3) and magnesium hydroxide (MgOH2), similar to that excreted by corals and artificially induced by electrolysis. This irregular and porous surface (see Figure 1) favours the adhesion of sessile marine benthos organisms that act as biofilters.

Figure 1: aspect of the calcareous coating at the end of the electrolysis phase.
To carry out the electrolysis, a metal electrode (cathode) is used, which will constitute the artificial reef when the electrolytic process is completed, and another iron electrode (anode) –although there may be two– which is placed in a concentric or parallel position, with a fixed and equidistant separation that guarantees an optimal flow of ions between the electrodes. To avoid contact between the electrodes, spacer elements made of non-conductive material, e.g. PVC, are placed between the electrodes (see Figure 2).
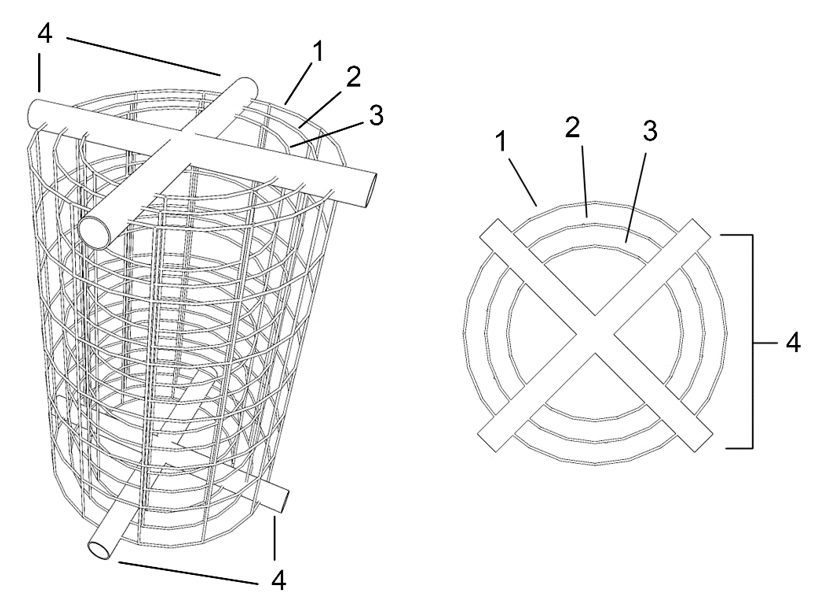
Figure 2: perspective and plan view of the electrolytic unit, showing the cathode without scale deposit (2), the external anode (1), the internal anode (3) and the non-conductive elements maintaining the concentric structures (4).
If only one anode is used, the inner anode (3) is dispensed with.
The preferred substrate for forming these artificial reefs is a lightweight metal mesh –preferably carbon steel– consisting of:
• Small diameter bars.
• Sheets.
• Plates.
• Beams.
• Etc.
These elements can be grouped together by assembling, welding or fixing with joining or stacking elements, and the mesh can have any geometry, shape and dimensions (see Figure 3).
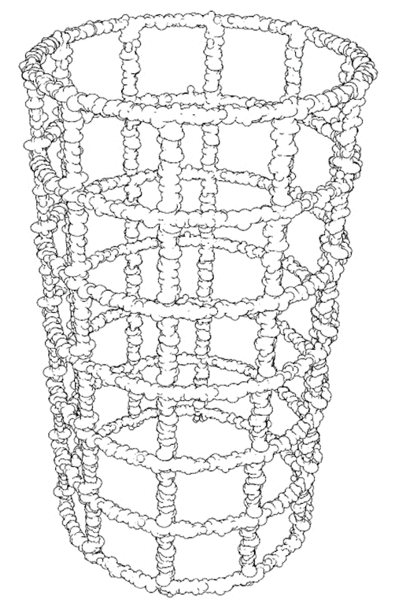
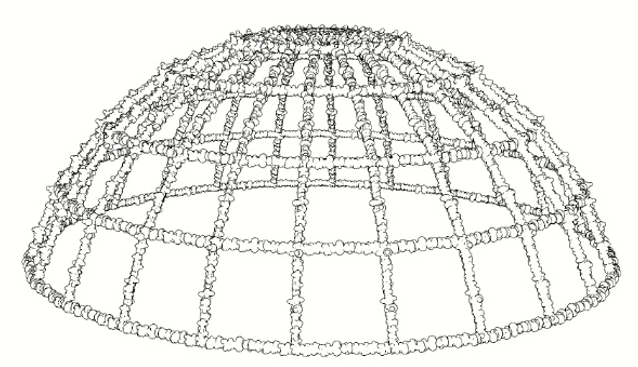
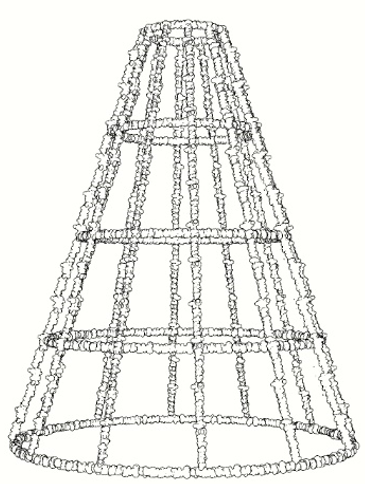
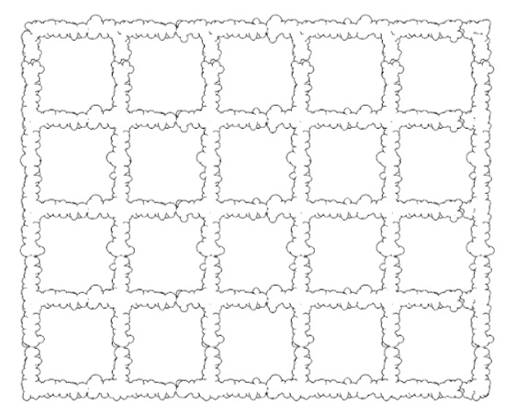
Figure 3: Different geometries of artificial reefs and underwater structures covered by calco-magnesian deposits, devoid of the outer and inner anodes and non-conductive elements.
By grouping several of these structures together –in a suitable shape and size– it is possible to build more complex modular structures (see Figure 4).
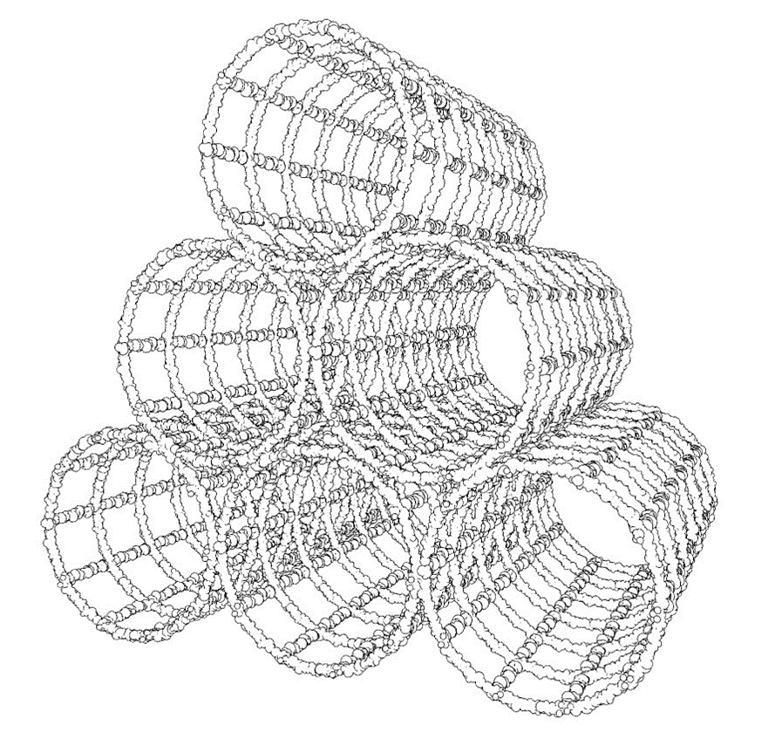
Figure 4: a cluster of six cylindrical modules that could be used as a biofiltration system after being coated with sessile marine benthic organisms.
To carry out the electrolytic process deposition, electrical wiring –shielded and insulated– is welded independently to the cathode and anode(s), and an external power supply is used to establish a continuous flow of electrical current between the electrodes when they are immersed in a conductive medium –saline liquid medium or seawater– both in pools and in large volumes of water with continuous movement and agitation –open sea, ports, salt marshes, etc. – (see Figure 5).
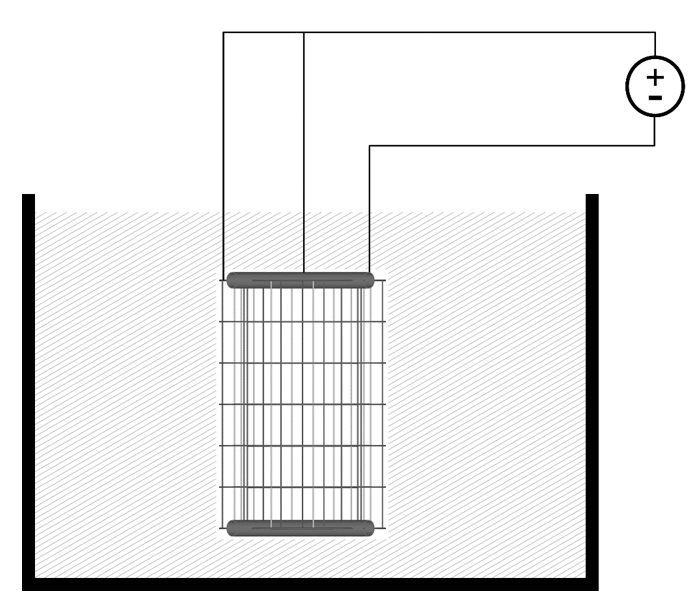
Figure 5: three-electrode electrolytic unit immersed in seawater or saline water during the electrolytic plating process.
The direct current supply can be provided by conventional grid transformation, by photovoltaic panels directly, or by the use of electric energy accumulators or batteries (see Figure 6).
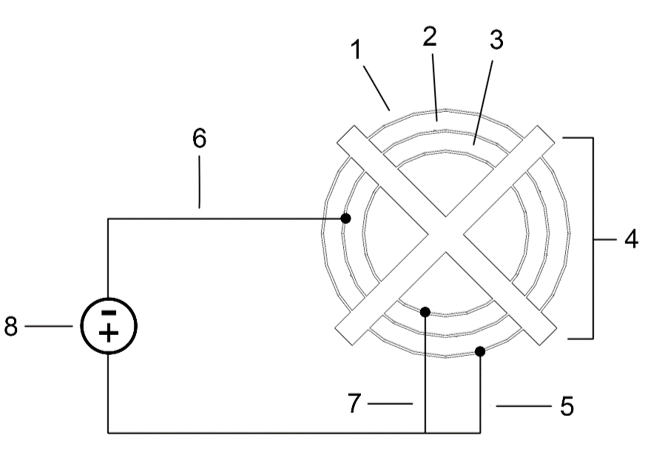
Figure 6: Electrical connections of the elements of the three-electrode electrolytic unit.
The electrolysis process is carried out at low current densities, and the thickness of the deposited stone layer can be controlled according to the intended applications of the system. The deposition rate is constant, and has a linear relationship to the time of application of the electric current (see Figure 7).
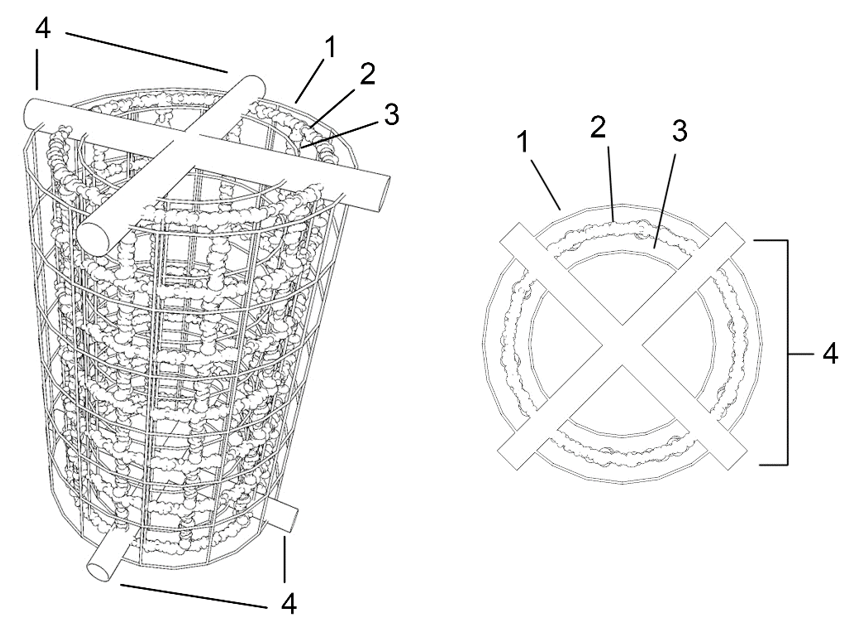
Figure 7: Perspective and plan view of the three-electrode electrolytic unit with the unitary modular unit coated with the calco-magnesian deposit.
During the electrolysis process, the anode(s) are partially dissolved in the form of iron cations (positively charged iron ions: Fe2+ or Fe3+), which serve as food for phytoplankton and form corrosion products that are harmless to the marine environment.
Once the calco-magnesian deposit formation stage is completed, the electrolytic unit is removed from the saline environment, the wiring, anode(s) and spacer elements are removed, thus obtaining the final structure ready for use –either individually or in combination with other–.
ADVANTAGES OF THE TECHNOLOGY
The main advantages of this method compared to other systems currently in existence for similar purposes are as follows:
1) The supports used have a very low weight, as they are made of metal mesh on which the electrolysis is carried out.
2) These systems can be easily transported, positioned and anchored due to their low weight.
3) The final structures can adopt any geometrical shape, and can subsequently form more complex underwater structures.
4) The manufacturing process can be carried out in-situ at the final location or in more controlled environments (e.g. swimming pools, port areas, salt pans or industrial sites). The latter option facilitates a more exhaustive control of the process.
5) The assembly can be carried out in-situ by assembly, welding, fixing with connecting elements or stacking of the necessary modular elements.
6) The electrolytic process avoids or reduces the environmental impact on the habitat, as chemical species beneficial to living organisms in the marine environment are generated during the process. In fact, the products of the anodic electrochemical reaction are iron ions (Fe2+ and Fe3+), species that favour the development of phytoplankton.
7) The large mesh spacing allows a greater homogeneity of the marine current lines, which favours the homogeneous growth of the calco-magnesian coating.
8) It allows a favourable interaction with the marine environment by facilitating the adhesion of species.
9) The free access and circulation of aquatic animals through the structure generates spaces that allow the protection and development of numerous living species.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The main innovation of this new system is the use of the same ferrous element in both electrodes (cathode and anode). This provides the following benefits:
• The generation of chlorine in gaseous form is avoided.
• Acidification of the liquid medium in the vicinity of the electrolysis zone is partially reduced.
• The oxidation of the anode releases positive iron ions (Fe2+ and Fe3+), and these favour the development of phytoplankton.
• These cations are innocuous elements and totally respectful of the ecosystem.
• With these systems, a greater diversity and recruitment of marine species is achieved in the structure, as they allow the free circulation of aquatic animals through the structure.
In addition, the electrolysis process is carried out using electrical current supplied by an external source, which allows the process of calcareous coating of the structural support to be accelerated and perfectly controlled.
A prototype has been successfully constructed (see Figure 8) to study the technical feasibility of this system for biofilter attachment (sessile macro-biofouling).

Figure 8: metal structure with the calcareous coating obtained by electrolysis.
This prototype (TRL=4) was placed in a real environment (Port of Alicante, Spain) during a three-month anchorage, and the conclusions obtained are as follows:
• 37 different species have been found.
• The carbonate substrate had a higher biodiversity and a more developed successional stage.
• Sponges and ascidians, and most bivalves (all of them are filter-feeding organisms) only occurred on the carbonate substrate, making it an optimal material for building biofilters.
These results allow us to evaluate carbonate electrolyte structures very positively as substrates with great potential as material for the construction of artificial reefs and underwater structures.
This technology finds its main application in the fields of:
1. Restoration of marine ecosystems through the construction of artificial coral reefs.
2. Mitigating the environmental impact of waste from grow-out cages in industrial aquaculture farms by anchoring structures that act as biofilters of organic matter (purification of marine waters).
3. Marine leisure and sustainable exploitation of coastal areas and the near-shore maritime fringe, through the construction of structures for recreational diving activities (underwater water parks, underwater aquatic parks, etc.
Companies interested in acquiring this technology for commercial exploitation through utility model licensing agreements are sought.
Company profile sought:
• Manufacturers of artificial marine reefs.
• Manufacturers of underwater structures for recreational diving or marine water purification.
This invention is protected by a utility model application:
• Title: “Sistema para la formación de arrecifes marinos artificiales y estructuras submarinas con recubrimiento calcáreo inducido por electrólisis”.
• Application number: U202130622.
• Application date: 26th March, 2021.
Construction and Architecture
Pollution and Environmental Impact
Marine Studies
Chemical Technology
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




