Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The research group is looking for companies interested in licensing the technology, developing R & D projects to optimize this initial idea and/or to adapt this development to their needs for a future commercial exploitation of the patent.

The current electric energy storage systems can be classified according the type of energy used. Thus, it can be considered systems using potential energy from compressed air (CAES, Compressed Air Energy Storage) or from pump-hydro (PHS, Pump-Hydro Storage), also using kinetic energy from flywheel (FWES, FlyWheel Energy Storage) or, finally, the most utilized ones where electrical energy is used.
These latter ones can be classified in two main types: supercapacitor systems and systems where electrical energy is stored in two reversible redox pairs. The charge capacity of these systems is defined by the mass of these reagents. This system can be represented by rechargeable batteries where reagents are static such as traditional lead-acid (P/A); nickel-cadmium system (Ni-Cd) and nickel metal hydride (Ni-MH), lithium-ion (Li-ion) or, redox flow batteries (RFB), in which solutions associated to redox pairs are pumped to negative and positive electrode as appropriate. For this reason, these solutions are defined as posilyte and negalyte. The system, which is described in our patent, uses hydrogen to perform the charge and discharge processes. Posilyte and negalyte is acified and basified in charge process respectively. In discharge process, neutralization energy of these solutions is used. It is important to note that, hydrogen is consumed and produced in charge and discharge processes. Thus, theoretical net balance is close to zero and therefore, external supply of hydrogen is very low or negligible.
The developed technology consists of an electric energy storage system using neutralization energy of two solutions, one acidic and the other alkaline, separated by an ionic interchange membrane and hydrogen production/ self-supply is our chain gear in the present system.
Next, a diagram of the configuration of the electric energy storage system is shown:
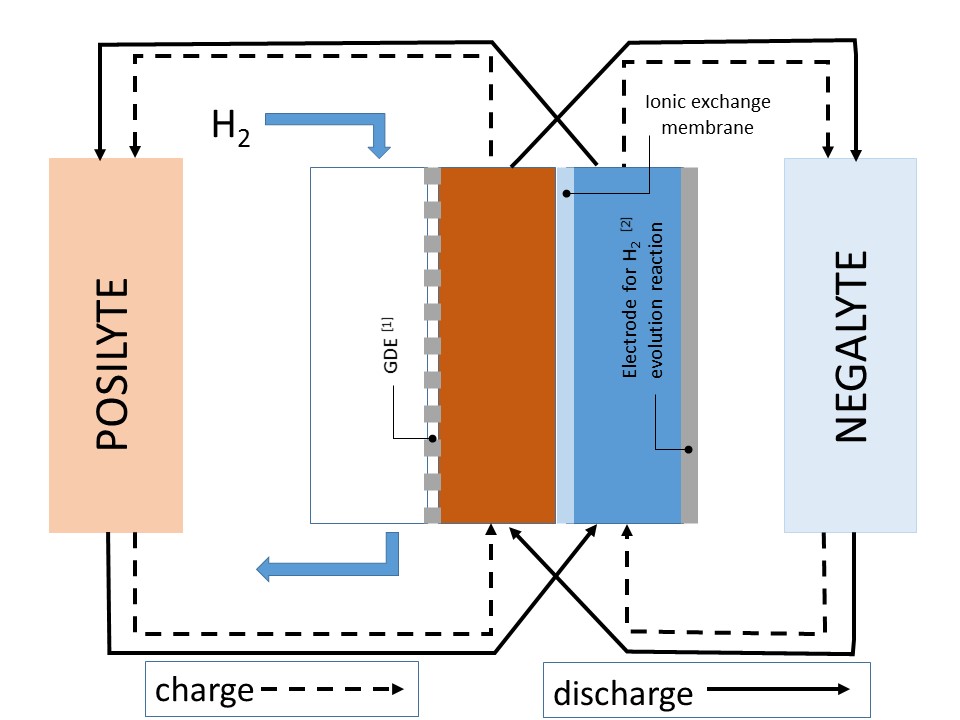
[1] GDE: Gas diffusion electrode
[2] Electrode catalysed using an appropriate metal.
The operation of this system is based in the potential difference appearing when two electrodes are submerged in two solutions with different pHs, one highly acidic and the other one highly alkaline. Both solutions have a support electrolyte in concentrated enough for ionic transport through membrane,
performed by support electrolyte ions. In this sense, hydrogen production / self-supply is the chain gear of the present system.
Hydrogen oxidation reaction to hydronium ions and hydronium ion or water reduction reaction to hydrogen is produced, both processes are very reversible. The acidity and basicity of starting solutions is decreased obtaining electric energy (discharge) or is increased providing electric energy (charge).
The reactions which are involved in charge and discharge processes are the following:
• Discharge process:
Negative electrode: ½ H2 + OH- → H2O + e
Positive electrode: H+ + e → ½ H2
H+ y OH- is consumed (in different electrodes, certainly) and H2O is globally produced.
• Charge process:
Negative electrode: H2O + e → ½ H2 + OH-
Positive electrode: ½ H2 → H + + e
Posilyte and negalyte are acidified and basified.
ADVANTAGES OF THE TECHNOLOGY
• High faradic efficiency in the process. Higher faradic efficiency and reversibility than other systems.
• It takes advantage of hydrogen self-supply.
• Simplicity of the system and utilization of simple and environmentally friendly substances.
• Cheaper reagents than those of other redox flow systems.
• High energy storage capacity inasmuch as it only depends on hydronium and hidroxyl ions concentration in the system.
• No imbalance of evolved redox pairs in the system is considered. Only pH adjustment system could be used if it necessary.
INNOVATIVE ASPECTS OF TECHNOLOGY
• The advantages of this technology are associated to innovative aspects of this technology.
• The own simplicity of the concept adds an undoubtable innovative character because this electric energy storage system is based in the use of “neutralization energy” of the system.
• The utilization of hydrogen production and self-supply is considered as chain gear which operates this system.
• Hydrochloric acid and sodium hydroxide solutions with sodium chloride are mainly used as reagents. All reagents are very cheap and environmentally friendly.
A proof of concept has been tested in a laboratory scale.
Electric energy generation and storage.
Companies interested in acquisition of this technology are looked for. In this case, a commercial prototype could be developed by:
• License agreements of the patent.
• R & D proyects.
This technology is protected by granted patent.
• Patent title: “Acid- base electrochemical flow battery (ABEFB)”.
• Application number: 201531141
• Application date: 31/07/2015
https://cvnet.cpd.ua.es/curriculum-breve/grp/en/electroquimica-aplicada-y-electrocatalisis/356
Materials and Nanotechnology
Chemical Technology
Transport and Automotive
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




