Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Materiales Carbonosos y Medio Ambiente (MCMA) research group at the University of Alicante has developed a new method to obtain high quality electrocatalytic powder materials from agricultural waste.
The procedure is very simple, it is easy to scale up to industrial level, it has a low manufacturing cost and the materials obtained are characterised by high durability and selectivity towards the oxygen reduction reaction, reasons why they may become very promising candidates to replace current commercial platinum-based catalysts for use them in fuel cells.
We are looking for companies interested in acquiring this technology for commercial exploitation.

The massive use of fossil fuels has led to an energy crisis that demands the evolution towards an economy based on renewable energies. Although important steps have been taken towards the decarbonisation of the energy system, there are significant limitations to its implementation in sectors such as transport, which is the second largest contributor to CO2 emissions and the main contributor to increase in greenhouse gas emissions in recent years.
The use of batteries in some transport sectors is not technologically feasible, so the development of technology for the generation and consumption of renewable energy through green hydrogen is essential. Moreover, hydrogen is a potential energy carrier because it can be produced from renewable energies, stored for long periods of time and then consumed to produce electricity. In this application, fuel cells play a very important role.
The consumption of green hydrogen via electrochemical fuel cells has a high efficiency compared to combustion. However, this is currently not a reality due to the large amount of platinum (a scarce and expensive noble metal) required in the catalyst (0.2-0.4 mgPt·cm-2) for the oxygen reduction reaction at the cathode. In addition, the agglomeration of the metal nanoparticles during use causes a significant reduction of the catalytic activity of the fuel cell with the working time, which shortens its lifetime.
The most developed fuel cells are low-temperature proton exchange polymer membrane fuel cells, although recently basic fuel cells are being developed, as the activity of non-noble metal-based materials is higher in an alkaline medium than in an acid medium.
Carbonaceous materials doped with nitrogen and metal sites have great potential in electrocatalysis, improving the catalytic activity in the oxygen reduction reaction. In addition, bimetallic species are of great interest, as they can show higher activities than the metals of which they are composed due to synergistic effects.
At current, development of carbonaceous materials for electrocatalytic applications using biomass waste as a precursor has a major limitation, as all these processes require catalyst washing processes in acidic media, water or organic solvents. These washing processes have a significant economic and environmental cost, which makes it difficult to scale them up industrially. In addition, more than one high temperature heat treatment is usually applied during their manufacture, which involves a significant energy and economic cost.
Therefore, none of the strategies known to date have solved the problem of obtaining low-cost catalysts based on agricultural residues (biomass) with sufficient catalytic activity and durability to replace existing platinum catalysts in the oxygen reduction reaction, and whose synthesis can be simply extended on an industrial scale.
In order to solve the problems described above, a new method has been developed for the synthesis of low-cost carbonaceous materials with excellent electrocatalytic properties using agricultural waste (biomass) for use them in fuel cells in an alkaline medium.
The procedure comprises the following steps:
1) Introduce the following compounds (in a given ratio) in an autoclave:
• The biomass precursor (ground to a specific particle size).
• A nitrogen precursor.
• A transition metal precursor (other than platinum group metals).
• Water as solvent (to achieve a homogeneous distribution between all the above compounds).
2) Place the autoclave in an oven at a certain temperature for a specific time.
3) Cool to room temperature.
4) Place the product obtained in the previous stage, homogeneously distributed, in a hermetically sealed oven.
5) Gradually, heat the oven to a certain temperature with a flow of inert gas.
6) Maintain the temperature reached in step 5) constant for a certain time.
7) Cool down to room temperature while maintaining the inert atmosphere.
8) After this process, the carbonaceous material is obtained in the form of fine powder (electrocatalysts).
The starting biomass can be any agricultural waste, for example: almond shells, olive pits, peach pits, wood, sawdust, cocoa shells, shellfish industry waste, etc.
The nitrogen precursor can be a nitrogen compound, such as: urea, melamine, dicyanediamide, etc.
Transition metal precursors are abundant in the earth's crust, and they can be metallic compounds, e.g. an inorganic salt or an organic salt of the metal, such as an iron oxalate, cobalt oxalate, nickel oxalate, etc. In no case, they are platinum group metals used, including: iridium, osmium, palladium, platinum, rhodium and ruthenium.
Nitrogen or argon may be used as an inert gas.
The electrocatalysts obtained by this procedure have specific surface areas greater than 500 m2·g-1.
The synthesis temperature has a very significant impact on the catalytic activity and selectivity of the carbonaceous compounds obtained, so that, by controlling this variable, catalytic activity values comparable to commercial electrocatalysts based on platinum nanoparticles can be achieved.
Similarly, it is key to use a specific nitrogen precursor to achieve high performance, obtaining carbonaceous compounds with activity and selectivity similar to commercial platinum catalysts.
Therefore, controlling all these factors is essential to obtain carbonaceous materials with excellent electrocatalytic properties, and the research group is an expert in this field.
The results obtained in some tests for these novel electrocatalysts are shown below (see Figures 1, 2, 3 and 4):
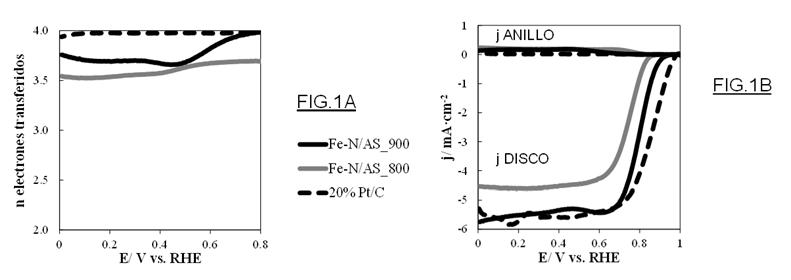
Figure 1: (A) Number of electrons transferred, and (B) Linear scanning voltammetry curves, at different temperatures.
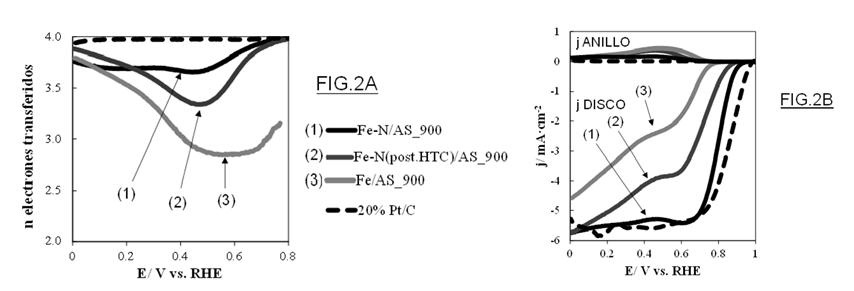
Figure 2: (A) Number of electrons transferred, and (B) Linear scanning voltammetry curves, modifying the nitrogen precursor.
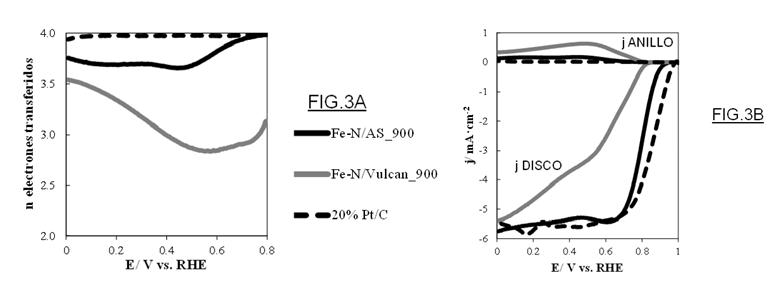
Figure 3: (A) Number of electrons transferred, and (B) Linear scanning voltammetry curves, using biomass or Vulcan carbon black.
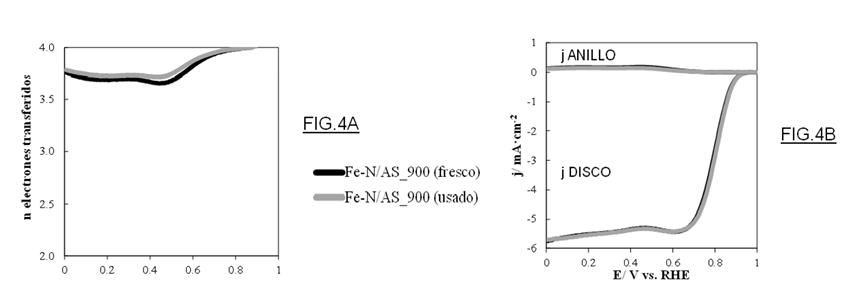
Figure 4: Durability study showing: (A) Number of electrons transferred, and (B) Linear scanning voltammetry curves, for fresh and used catalyst after 200 working cycles.
ADVANTAGES OF THE TECHNOLOGY
The main advantages of this novel procedure are listed below:
1) It is easier and it has fewer steps than currently used procedures.
2) Porosity development and surface functionalisation is carried out in a single step.
3) It does not require special equipment: the equipment used is commercially available and affordable for any laboratory or industry.
4) Catalysts are obtained in fine powder form, so they are easy to handle and to disperse in aqueous media at room temperature, which facilitates their conformation as electrodes. In addition, the prepared suspensions are stable over time.
5) The precursors used are very cheap and abundant, among them:
• Agricultural waste or any type of vegetable biomass (almond shells, coconut shells, cocoa shells, olive pits, peach pits, date pits, plum pits, etc.).
• Transition metals such as: iron, cobalt, nickel, etc.
6) Platinum group metals are not used, including: iridium, osmium, palladium, platinum, rhodium and ruthenium.
7) The final cost of the prepared materials is lower than commercial platinum-based catalysts.
8) The process can be easily scaled up to industrial level.
9) The carbonaceous materials obtained have high physico-chemical and mechanical properties:
• They are very stable (as commercial catalysts).
• Electrocatalytic activities similar to commercial platinum electrocatalysts are achieved in the oxygen reduction reaction in an alkaline medium.
• They show excellent durability after many reaction cycles (200 cycles).
• They are very robust, as they do not inactivate after the demanding durability tests to which they have been tested.
• They are characterised by their high selectivity to form water by the mechanism of transferring four electrons per oxygen molecule.
• The generation of by-products such as hydrogen peroxide, which limits energy yield and is damaging to the working of fuel cells, is prevented.
• They have specific surface areas greater than 500 m2·g-1.
10) Conventional activating agents are not used.
11) The carbonaceous materials obtained do not contain residues (usually formed in the conventional activation process).
12) Only a single stage at high temperature is required.
13) No subsequent catalyst washing steps are required.
14) The process has a low environmental impact. It is sustainable and environmentally friendly.
15) It reduces dependence on fossil fuels and it reduces the carbon footprint, thus contributing to the transition towards an economy based on renewable energies.
16) The synthesis method is versatile and it can be applied to other electrochemical reactions of interest by modifying the metallic precursor or by introducing other metals in the form of alloys.
17) The synthesis method has a high yield.
18) These materials are well suited to replace platinum electrocatalysts in low-temperature fuel cells in alkaline media.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The main innovation concerns the use of agricultural waste (biomass) to obtain low-cost carbonaceous materials with excellent electrocatalytic properties to replace current commercial platinum-based catalysts in the oxygen reduction reaction in an alkaline medium in fuel cells.
Furthermore, the present invention differs from current synthesis methods in two fundamental aspects:
1) Conventional activating agents involving subsequent washing steps are not employed. Thus, this invention provides a simple method of synthesis.
2) The chemical activation of the carbonaceous precursor and the incorporation of the active sites is carried out during the same thermal treatment, using just the right amount of the metal precursors and nitrogen.
On the other hand, in contrast to this invention, current synthesis methods require several washing steps, which increases the price of catalysts and contributes to a deterioration of the environment.
Through the appropriate selection of metal precursors (e.g. metals such as iron), nitrogen and appropriate biomass residues, catalysts for the oxygen reduction reaction in alkaline media can be obtained with similar features to commercial platinum catalysts.
Moreover, it is important to note that in this novel synthesis procedure:
• No washing steps (with water, acid solutions or organic solvents) are required after heat treatment, which it reduces the environmental impact and manufacturing cost.
• Only a single heat treatment at high temperature is required.
These novel electrocatalysts (see Image 1) have been successfully synthesised at laboratory level. This technology is at TRL = 4 (Technological Readiness Level).

Image 1: Synthesised electrocatalysts in fine powder form and the supports on which they are deposited, respectively.
The kinetic parameters obtained for these new electrocatalysts for the oxygen reduction reaction in alkaline medium obtained from almond shells, whose values are similar to those obtained for commercial platinum electrocatalysts, are listed below:
• Reaction onset potential = 0.92 V vs. RHE.
• Half-wave potential = 0.79 V vs. RHE.
• Boundary current density similar to platinum-based electrocatalyst.
• Number of electrons at useful fuel cell potentials (0.6-1.0 V vs. RHE) between 3.8-4.0 RRDE (corresponding to the generation of practically water as the only product, avoiding the production of by-products such as hydrogen peroxide (less than 5%) which limits the energy yield and is detrimental to the working of the fuel cells).
As can be observed in the tests carried out, very promising results have been obtained in the oxygen reduction reaction in alkaline medium, indicating that the carbonaceous materials obtained from biomass waste show excellent catalytic activity and durability comparable to commercial platinum-based catalysts, which makes them very suitable for use them in fuel cells.
Moreover, materials obtained have been characterised using different techniques, such as transmission electron microscopy, X-ray spectroscopy and Raman spectroscopy, which has allowed us to know their structure and composition.
This invention is part of the energy and circular economy sectors. Specifically, it focuses on the use of agricultural waste (biomass) to obtain high-performance carbonaceous materials (electrocatalysts) for industries that manufacture or use fuel cells.
This technology allows obtaining carbonaceous materials free of platinum group metals, including iridium, osmium, palladium, platinum, rhodium and ruthenium, for application as excellent electrocatalysts in the oxygen reduction reaction at the cathode of the alkaline polymer membrane fuel cell.
These novel electrocatalysts are potential candidates to replace commercial platinum electrocatalysts in the oxygen reduction reaction in alkaline media, thus decreasing the overall cost of low-temperature fuel cells.
Sectors of interest:
1. Energy: this technology can be used for the production and storage of renewable energy (distributed and stationary) using green hydrogen, contributing to the decarbonisation of the energy system and the reduction of greenhouse gas emissions.
2. Transport: the technology can be used in the manufacture of fuel cells for electric vehicles, contributing to the transition towards more sustainable and environmentally friendly transport.
3. Chemical industry: the technology can be used in the synthesis of chemical products of commercial interest.
4. Pharmaceutical industry: the technology can be used in the synthesis of pharmacological molecules of interest to the health sector.
5. Waste management: the technology can be used in the valuation of biomass waste, which it contributes to the reduction of the amount of waste sent to landfills and the reduction of the carbon footprint.
We are looking for companies interested in acquiring this technology for commercial exploitation through:
• Patent licensing agreements.
• Development of new applications.
• Agreements regarding technology and knowledge transfer.
Company profile sought:
• Manufacturers of catalysts and electrocatalysts for fuel cells.
This invention is protected through patent application:
• Title of the patent: "Procedimiento de síntesis de un material carbonoso y su uso como electrocatalizador, especialmente para la reducción de oxígeno".
• Application number: P202330583.
• Application date: 12th July, 2023.
Pollution and Environmental Impact
Chemical Technology
Transport and Automotive

Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




