Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Joint Research Unit in Biomedical Design and Manufacturing (BioFab), consisting of researchers from the University of Alicante and the Institute for Health and Biomedical Research of Alicante (ISABIAL), has developed a simulator of the middle meningeal artery to improve training in the surgical practice of chronic subdural hematomas (CSDH) with embolizing substances. The system, manufactured by 3D printing, replicates the anatomy of the meningeal artery in a realistic and calibrated way, providing the medical team with experience and testing both the approach and the use of the different embolizing substances, prior to the intervention with real patients.
BioFab is looking for medical device companies or healthcare entities interested in validating the simulator or in the design and manufacture of other new devices.

Embolisation is a surgical procedure that involves blocking the blood flow of certain vessels using special substances to treat various conditions. In interventional neuroradiology, this approach is increasingly used in the middle meningeal artery as a non-invasive alternative to treat chronic subdural haematomas. Despite the growing number of centres and specialists adopting this treatment, the majority still opt for the use of the cerebral trephine, as the skill acquisition and technical training to embolise the middle meningeal artery is complex and costly.
The use of animal models is falling into disuse, while the available simulators focus mainly on peripheral areas of the human body, such as the kidneys and the arteries of the digestive system. There are also simplified and aneurysm-focused models or virtual simulators that are unreliable for real practice. Therefore, at present there are no simulators based on the specific anatomy of the middle meningeal artery, which means that the training of healthcare professionals is very deficient.
In view of the above, an embolising substance simulator is proposed which offers a simple (see Figure 1) but efficient solution to simulate the anatomy of the middle meningeal artery, generating tactile sensations of real practice.
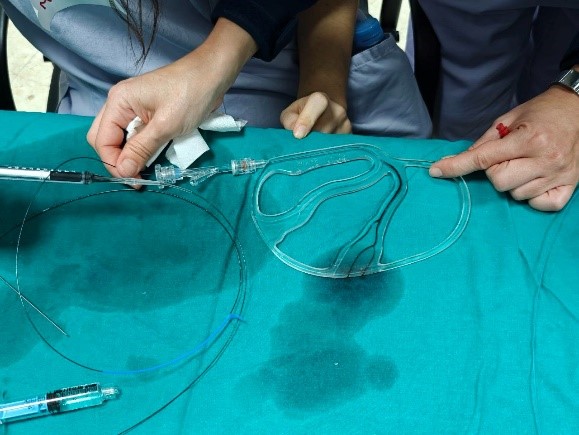
Figure 1: Embolisation test with the simulator
This invention allows the anatomy of the middle meningeal artery to be reliably and realistically reproduced. This facilitates more detailed training and preparation for embolisations of the median meningeal artery, allowing the medical team to make more accurate decisions about the appropriate embolising substance and the best possible approach.
It consists of two parts:
• A branched model based on the geometry of the middle meningeal, with inner lumens of up to 0.5 mm replicating actual anatomical internal tortuosities. It is manufactured by stereolithography (SLA) from a transparent and radiolucent material, so it can be used under fluoroscopy to replicate the same conditions as during surgery.
• A rigid base manufactured by 3D printing (FDM) that is attached to the branched model and gives it greater stability and protection against knocks and falls.
It should be noted that additive manufacturing (commonly known as 3D printing), especially stereolithography (SLA), has become a productive alternative in traditional industry and offers customised solutions to healthcare problems such as the present invention.
MAIN ADVANTAGES OF THE TECHNOLOGY
Some advantages are worth noting:
• Great realism with real anatomy, even replicating the complicated internal tortuosities that other commercial models simplify (0.5 mm internal lumens).
• Improved physician training (current embolising simulators focus on aneurysms) so they can make more accurate decisions about the appropriate embolising substance and the best possible approach.
• Made of a transparent material that allows healthcare staff to directly observe the behaviour of the embolising substance.
• Facilitates the evaluation of the behaviour of new embolising substances under development in a quasi-real environment.
INNOVATIVE ASPECTS
3D printing and three-dimensional models are being used in a large number of sectors as production technologies for small series as they offer great flexibility and the possibility of customisation.
Therefore, it is a manufacturing process that is characterised by the low cost of the materials and equipment required, as well as the possibility of being produced anywhere in the world with the consequent savings in logistics and distribution.
In the case of the present invention, it has the limitation of being single-use because once the formation with the embolising substances has been carried out, the inside of the duct becomes clogged and can no longer be used for other formations. Furthermore, numerous hours of post-processing are required to obtain a functional prototype.
A prototype has been developed with the participation of different medical specialists who have tested and adjusted the dimensions and performance of the simulator (see Figure 2). Therefore, a device that perfectly fulfils the objectives sought has been achieved.

Figure 2: Simulator view
It is primarily aimed at the health technology and innovation sector with the objective of improving medical training and surgical planning for specialists.
Companies interested in acquiring this technology for its commercial exploitation through patent licensing agreements are sought, as well as healthcare entities interested in validating the simulator or in the design and manufacture of other new devices.
This technology is protected by patent application:
• Patent title: “Simulador de sustancias embolizantes con la anatomía de la arteria meníngea media”
• Application number: P202431095
• Application date: 24/12/2024
Engineering, Robotics and Automation
Medicine and Health
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




