Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The research group has developed a highly enantioselective process catalysed by small amounts of a chiral complex formed by a silver(I) salt and a chiral phosphoramidite. This method allows the preparation of enantioenriched polysubstituted proline derivatives after a three-step sequence. These prolines are widely used for multiple purposes, but the most important activity is based on the high efficiency against the hepatitis C virus. The administration of these drugs in mammalians is beneficial because the risks of the multiple side effects are reduced and the required dose is very small, unlike the mixture of the pharmaceuticals employed in the current therapy. This research group asks for companies ready to acquire this methodology for a future exploitation.

An estimated 3% of the global human population is infected by hepatitis C virus, an infection that often leads to cirrhosis, hepatocellular carcinoma, and liver failure in later life. It has been estimated that of those currently infected, 20% and 4% are likely to develop liver cirrhosis and liver cancer, respectively, in the next decade. The current gold standard therapies are based upon the use of pegylated interferon-¿ in combination with ribavirin. These therapies provide a sustained virological response in around 50% of patients infected with genotype 1 and have the disadvantage of frequent and severe side effects, such as fever, chills, headache, muscle ache, fatigue, nausea, loss of appetite, depression, anxiety, irritability, insomnia, mental confusion, suicide, neutropenia, thrombocytopenia, colitis, heart and thyroid problems, some lung disorders, autoimmune diseases, vision problems, hair loss, etc. The development of new therapies to treat hepatitis C virus infection effectively is, therefore, of paramount importance and is currently an intensive area of research.
The hepatitis C virus is a small, enveloped virus, the genome of which is a 9.5 kb single-stranded RNA that encodes for a single large polyprotein of 3010-3030 amino acids. This polyprotein is processed by cellular signal peptidases to produce the structural viral proteins, whereas viral proteases (NS2, NS3) are responsible for the production of mature non-structural proteins. These include the RNA-dependent RNA polymerase (RdRp, NS5B), which synthesises the new viral RNA strands. It is a well characterised enzyme, essential for viral replication, with no known mammalian equivalent and thus represents an attractive target for the development of novel anti-hepatitis C virus agents.
Recently, it has been disclosed the acyl polysubstituted pyrrolidine series identified through a high throughput screening program against the enzyme. The interaction drug-enzyme domain is well known and are fully characterised.
These pyrrolidine families have been obtained through 1,3-dipolar cycloadditions run at high temperatures and employing metallo-dipoles obtained from alkaline or transition metals. In all cases, the final products were achieved as racemic mixtures or as mixture of diastereoisomers, so an optical resolution with a phosphoric acid derived from 1,1’-binaphth-2,2’-ol or by using semi-preparative chiral HPLC, are frequently employing for the isolation of pure enantiomeric forms.
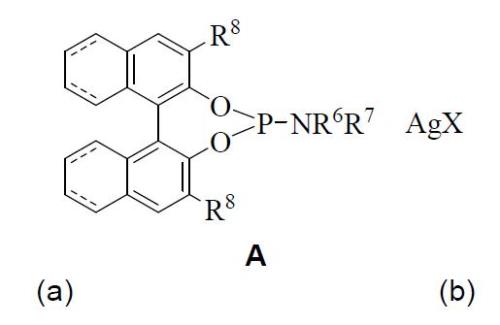
The most relevant features of this complex A are: 1) is the first described and published complex formed by a phosphoramidite unit (a) and a silver(I) salt (b); 2) R6 can be an hydrogen atom, alkyl radical (C1-C6), aryl group (C5-C14) or an alkylaryl substituent; 3) R8 can be an alkyl group (C1-C6), alkoxyalkyl, (dialkylamino)alkyl (C1-C6) or aryl; 4) R7 can be hydrogen or an alkyl (C1-C6), aryl (C5-C14) or arylalkyl groups; 5) X is selected from fluoride, acetate, perchlorate, trifluoromethanesulfonate, tetrafluoroborate, nitrate, hexafluorophosphate o hexafluoroantimoniate; and 6) (---) represents an optional chemical bond, which can be present or not.
It was also developed an optimised procedure where the new complex B could be obtained using a similar method but varying the stoichiometry of the reagents. It is composed by two equivalents of a chiral phosphoramidite (a) and an equivalent of silver(I) salt (b). The nature of R6-R8 and X groups has been described previously.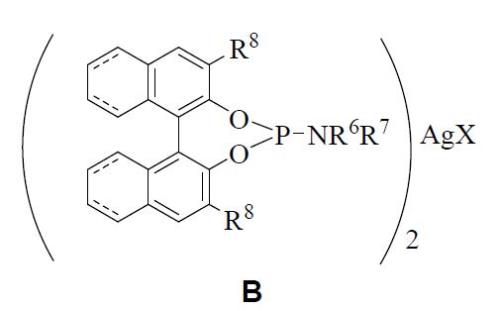
Both chiral complexes (A y B) are prepared by mixing one or two equivalents of chiral phosphoramidite (a), respectively, together with one equivalent of silver(I) salt (b) in the presence of an organic solvent, at room temperature and avoiding the light exposure.
The catalysed 1,3-dipolar cycloaddition between azomethine ylides, generated from iminoesters (III), and acrylates (IV) has been also optimised using substoichiometric amounts of the mentioned chiral silver complexes A and B.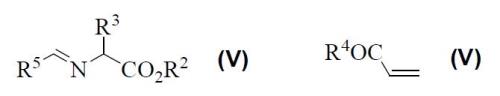
In the case of compounds (III) and (IV) R2 is and alkyl group (C1-C6), R3 is an alkyl (C1-C6) or arylalkyl group, R4 is a selected radical from alkoxy, alkylamino, arylamino, or dialkylamino, whilst R5 is a heteroaryl substituent. In this way, a series of intermediates and final products possessing similar structures (I) or (II) are obtained.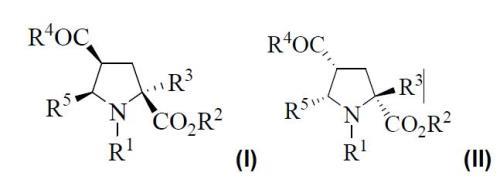
R1 and R5 are: R1 hydrogen, aroyl, R2 hydrogen or alkyl group (C1-C6), R3 alkyl (C1-C6) or arylalkyl, R4 can be hydroxy, alkoxy, alkylamino, arylamino or dialkylamino, y R5 heteroaryl group.
The enantiomerically enriched antiviral compounds derived from proline posses a general structure (I) or (II), where R1 is aroyl, R2 is hydrogen, R3 is an alkyl (C1-C6) or arylalkyl group, R4 is hydroxy, alkylamino, arylamino or dialkylamino, and R5 is a heteroaryl group.
The presence of the aroyl group in the final antiviral molecule is crucial for ensuring the inhibitory activity against the mentioned virus of the hepatitis C. The atom array of this substituent will allow the increment of the permeability of these drugs through cellular membranes.
Compound C has been also prepared.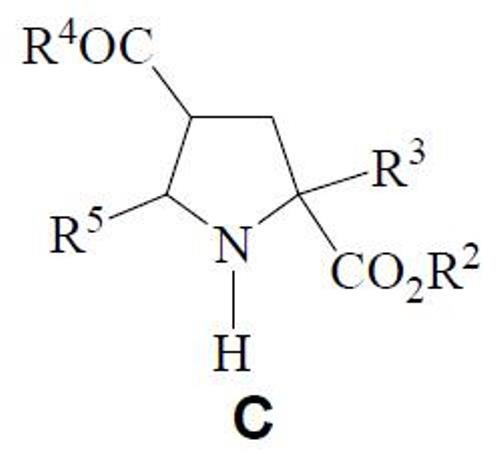
Here, R2 is an alkyl group (C1-C6), R3 is an alkyl (C1-C6) or arylalkyl group, R4 is an alkoxy, alkylamino, arylamino or dialkylamino, and R5 is a heteroaryl group.
The final absolute configurations of the C structures are: C(I)=(2S,4S,5R) and C(II)=(2R,4R,5S):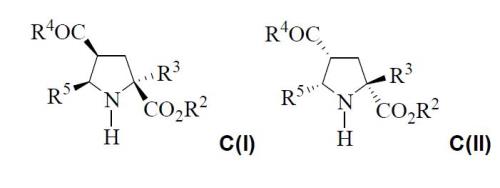
General structures D can be easily obtained from compound C. In this case R1 is aroyl and R2-R5 such as it was described previously. 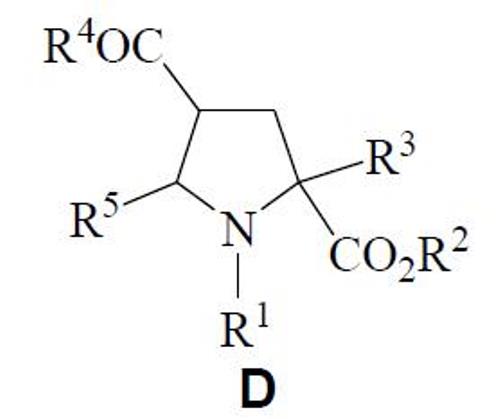
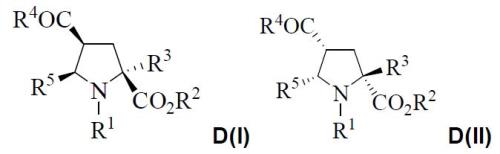
The absolute configurations of each stereogenic centre are the following: D(I)=(2S,4S,5R) and D(II)=(2R,4R,5S):
Finally, it has been elaborated a compound whose general structure obeys to E. The analogous compounds D are their precursors, where R1 is aroyl, whilst R3 and R5 have been detailed before.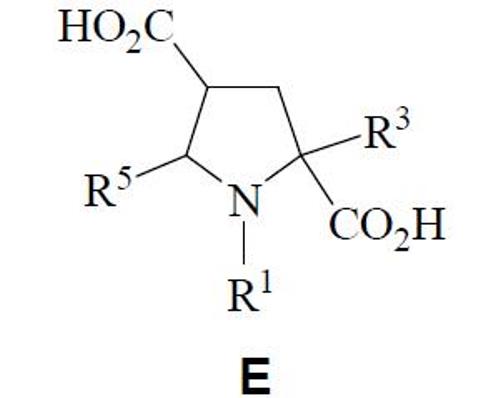
The absolute configuration of each stereocentre of products E are: E(I)=(2S,4S,5R) and E(II)=(2R,4R,5S): 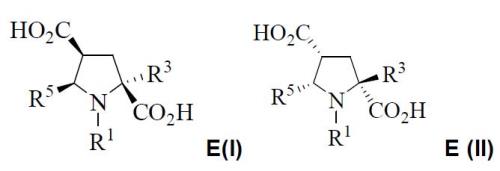
MAIN ADVANTAGES:
• The processes are highly enantioselectives. The final antiviral agents are obtained with very good ee’s.
• The chemical yields are very good, and the purity of the compounds is very high.
• The procedure is efficient and reproducible.
INNOVATION FEATURES
At this moment, this methodology is the unique dealing with the production of these antiviral agents in an highly enantioselective manner. This enantioselective synthesis is very reliable, reproducible and allows to react very bulky starting materials giving high chemical yields. This method is a clear example of the atom economy.
The general process has been optimised in a laboratory scale after multiple tests and structural modifications. The results are:
¿ High purities (more than 97%).
¿ High chemical yields (more than 70%).
¿ High enantiomeric ratios (at least 94:6 re).
The Pilot Plant of the Department of Organic Chemistry of the University of Alicante offers many possibilities to implement and perform this synthesis in an industrial scale. It is fully equipped an ready to develop the production in a range between milligrams and multi-kilo batches.
The facilities of this pilot plant are numerous, for example, the coverage of the production scale allows reactions up to 300 L, multiple chemical and physical treatments, storage, analysis, and the full control of the process. The pilot plant operates under certified Norma ISO 9001, maintaining the cGMPs (current Good Manufacturing Procedures).
PHARMACEUTICAL COMPANIES: they represent a very promising therapy against the proliferation and development of the virus responsible of the hepatitis C. Their efficiency and biological potential has been demonstrated in infected mice IC50= 0.3-0.5 ¿M, (even lower according to the new structures published in the literature).
The research group is looking for companies in acquiring the above described technology for a future exploitation. For that reason the group is ready to deal whatever kind of technological transfer under the supervision of the University of Alicante.
The technology is protected according to a patent application:
• International Publication Number: WO2009/121989 A1.
• International Publication Date: 08.10.2009.
Medicine and Health
Chemical Technology
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




