Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The research group "Atomic - mass spectroscopy and analytical chemistry under extreme conditions" of the University of Alicante has developed a method for the diagnosis and prevention of colorectal cancer (CRC). More specifically, an accurate, simple, sensitive and efficient method of extraction and analysis of volatile organic compounds (VOCs) for application as a non-invasive screening test of CRC by stool analysis has been developed.
This method is non-invasive, fast, reliable, economical, highly sensitive and for use in a wide range of patients, so that it can also eliminate or reduce the number of false positives and false negatives.
The technology is developed at laboratory level and is protected by a patent application.
Companies interested in its commercial exploitation are sought.
Colorectal cancer (CRC) is one of the leading causes of cancer death worldwide. Current methods of detecting CRC can be invasive, such as colonoscopies, or non-invasive, such as the faecal occult blood test (FOBT). Although these screening tests have helped to reduce mortality, their performance is not optimal. The FOBT has a substantial number of false negatives and as a consequence a significant number of missed diagnoses of CRC. In addition, a significant percentage of healthy participants who are screened in the population receive a false positive result, which in turn leads to the unnecessary use of colonoscopies. Colonoscopies are an invasive technique, with a risk of complications (bleeding or perforation) as well as a high cost.
Today there are two types of techniques focused on the study of volatile organic compounds (VOCs) for future application in CRC detection; analytical techniques and pattern recognition technologies. Analytical techniques allow the detection of alterations in the presence and concentration of specific molecules, while pattern recognition technologies are non-specific, meaning that they are not selective to a given compound, but to a group of compounds.
There are multiple publications that have provided studies that relate certain biomarkers, some of them VOCs, with the probability of a subject suffering from CRC through different methods. However, none of them have so far provided a method, which is fast, reliable, economical and with high sensitivity, to detect and quantify VOCs that can be useful as biomarkers and at the same time allow the development of a screening method that can reduce or eliminate false positives and false negatives in the detection of CRC.
Therefore, there is a need to develop highly sensitive VOC analysis apparatus and methods that can also quantify VOCs from complex samples such as solid, semi-solid or directly from faeces samples and that are also fast, reliable, sensitive and economical.
The research group of "Atomic - mass spectroscopy and analytical chemistry under extreme conditions" of the University of Alicante has developed a device adapted for the qualitative and quantitative analysis of volatile organic compounds (VOCs) in solid and/or semi-solid samples, which consists of the following three elements (Figure 1):
a) a headspace adsorptive magnetic extraction device comprising an inert container for depositing the sample. The container comprises a lid and two magnets, one located in the lower part of the lid, which comprises a magnetic sorbent containing a nanomaterial with graphite oxide and iron oxide supported on the magnet, and; another located in the upper part of the lid. This device is configured to be coupled with the next one;
b) a thermal desorption system coupled to a gas chromatograph-mass spectrometer that provides a qualitative analysis of VOCs, relating the position of the peaks and their retention time to the identification of VOCs, and quantitative analysis, evaluating and calculating the area of each peak;
c) a mass spectrometer that provides qualitative and quantitative analysis of VOCs as a function of the mass to load ratio of VOCs.
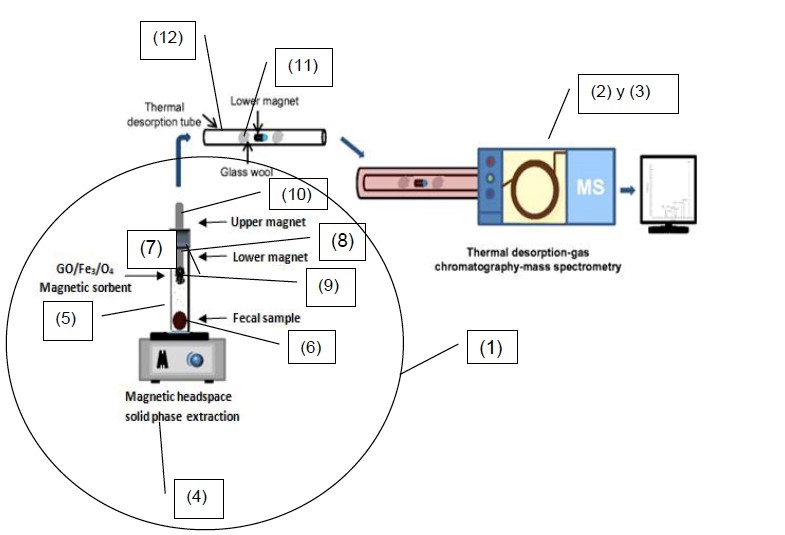
Figure 1. Schematic diagram of the devices used to carry out the analysis, being (1) headspace adsorptive magnetic extraction device; (2) gas chromatograph; (3) mass spectrometer; (4) apparatus for applying temperature and producing magnetic agitation; (5) inert container; (6) solid/semisolid sample; (7) lid; (8) magnet; (9) sorbent supported on the magnet; (10) magnet; (11) glass wool; (12) glass tube.
The headspace adsorptive magnetic extraction device allows the volatilisation of VOCs from a sample, subjected to a certain temperature. The volatile compounds in the headspace are subsequently retained in an adsorbent trap, which is then desorbed and injected for separation and detection by gas chromatography-mass spectrometry.
This apparatus allows the extraction, identification and quantification of VOCs (P-cresol, 1H-indol, 3(4H)-dibenzofuranone or tetrahydrofolate) from stool samples, known as biomarkers in subjects suffering from CRC, or in subjects who are predisposed to suffering from CRC, to provide a prognosis of the subject's condition or to provide a negative prognosis.
Therefore, this device can be used as a rapid, effective, selective and non-invasive ex vivo diagnostic method of CRC in a very large number of subjects. To do so, the following steps should be followed:
I. Obtain a stool sample from the subject;
II. Extract at least one VOC contained in the sample and identify and quantify the concentration of this VOC with the chromatograph through its retention time and mass spectrum;
III. To compare the concentration of at least one VOC with the concentration of a reference of the characteristic compound in a non-cancer individual sample, where the increase or decrease in the concentration of the biomarker compared to the reference is indicative that the subject is suffering from, or has a predisposition to, cancer, or provides a negative prognosis of the subject's condition.
A typical chromatogram of a subject's ex vivo sample may contain all four of the biomarker compounds cited, in both CRC and healthy control patients.
In summary, this invention has proven to be reliable, sensitive, reproducible, rapid and useful for reducing the number of false negatives or positives, for the diagnosis of colorectal cancer (CRC) in a subject suffering from cancer, or for the predisposition to it, or provides a prognosis of the subject's condition and/or CRC biomarkers or a negative prognosis of such condition.
MAIN ADVANTAGES OF THE TECHNOLOGY
The main advantages of the technology described are as follows:
• The device developed allows a reliable, effective, reproducible and fast analysis of VOCs (biomarker compounds) in solid and/or semi-solid samples.
• It has good sensitivity and selectivity.
• It is a non-invasive ex vivo diagnostic methodology for CRC.
• Useful for a very large number of subjects.
• Environmentally friendly use.
• The sorbent used can be reused after a stage after adequate cleaning, which improves its economic profitability and its use at a commercial level.
INNOVATIVE ASPECTS
The main innovative aspect of the technology is the fact that, until now, 3(4H)-dibenzofuranone had not been identified as a possible biomarker related to CRC or as a biomarker in subjects suffering from CRC.
The technology is developed at laboratory scale and has been used for the diagnosis of CRC in stool samples.
In this sense, in the analysis carried out, the research group observed that the increase in the concentration of the three biomarkers, 3(4H)-dibenzofuranone, p-cresol or tetrahydrofolate, in a sample of a subject to be evaluated compared with the sample of a healthy subject indicated the presence or the beginning of the disease or the probability of suffering from it or provided a negative prognosis of the subject's condition. Whereas, a decrease in the concentration of the biomarker 1H-indol in a sample of a test subject compared to a sample of a healthy subject indicated the presence or onset of the disease or the probability of having the disease or provided a negative prognosis of the subject's condition.
The method had a very good sensitivity (83% for both p-cresol and 3(4H)-dibenzofuranone) and specificity (80% for p-cresol; and 74% for 3(4H)-dibenzofuranone).
The technology described can be used as a method of diagnosis and prevention of CRC. More specifically, this technology is useful to extract and perform accurate, simple, sensitive and effective analysis of VOCs for application as a non-invasive screening test for CRC.
Partners sought:
Companies interested in acquiring this technology for commercial exploitation through:
• Patent license agreements.
• R&D projects to develop new applications for other types of diagnostics.
Company profile sought:
• Medical diagnostic laboratories
• Analytical instrumentation companies
This technology is protected by patent:
• Title of the patent: "Apparatus and methods for the diagnosis of colorectal cancer".
• Application number: P202030487
• Concesion date: 25 Nov 2022
Medicine and Health
Chemical Technology
Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959





