Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The Institute of Organic Synthesis and the Institute of Water and Environmental Sciences, belonging to the University of Alicante, have jointly developed an extractant mixture formed by the combination of a process ionic liquid (TSIL) and an ionic liquid (IL) that allows the selective and efficient extraction of metals of the f series of the periodic table (lanthanides and actinides) with respect to other metals of the s, p and/or d series. This technology is characterised by the fact that the extractant mixture can be reused in new extraction cycles without losing effectiveness, which represents a great advance in sustainability and environmental protection.
This novel formulation can be applied industrially in areas such as mining, nuclear chemistry, nuclear medicine and nuclear waste treatment.
Companies interested in acquiring this technology for commercial exploitation through patent licensing agreements are being sought.

The inner transition elements occupy the positions in the periodic table between the elements lanthanum (Z=57) and hafnium (Z=72), and are called lanthanides or lanthanoids, and between actinium (Z=89) and rutherfordium (Z=104), and are called actinides or actinoids.
All these elements are also called f-block or f-series elements, and are characterised by the fact that they are rare and widely dispersed in the earth's crust. The separation of an f-block element (or elements) from other s-, p- and/or d-block metals is a difficult, tedious and economically very costly task.
In general, lanthanides are used as very effective catalysts in industrial chemical processes. In fact, they are necessary and indispensable components in more than 200 high-tech consumer products in a wide range of applications, such as mobile phones, hard disks, electric and hybrid vehicles, flat screen monitors and televisions, in optics, in military defence applications (manufacture of electronic displays, guidance systems, lasers, radar and sonar systems), etc.
On the other hand, actinides are radioactive and release energy after their corresponding decay chains. Uranium and thorium (natural) and plutonium (artificially produced) are the most abundant actinides on Earth, and are mainly used in nuclear reactors, nuclear weapons and nuclear medicine.
The methods used to separate and obtain f-block elements free of other s-, p- and d-series metals are numerous, and most of them are based on liquid-liquid extraction procedures. The use of Ionic Liquids (IL) has been fundamental in many examples of selective chemical separation, however, the use of Task Specific Ionic Liquids (TSIL) combined with Ionic Liquids (IL) has not been used as frequently.
In any case, to date, no procedure has been carried out to recover the TSIL-IL extractant system, but only studies have been carried out to recover the metal (stripping) at the end of the extraction process.
Therefore, there is a need to obtain an extractant formulation and a procedure that allows the selective extraction of the internal transition elements (lanthanides and actinides) from the rest of the metallic elements of the s, p and d series, and that can also be used in repeated extractive cycles without losing selectivity or effectiveness.
In order to solve the problems described above, an extractant mixture consisting of a TSIL process ionic liquid (see chemical formula in Figure 1) dissolved in an IL ionic liquid (see chemical formula in Figure 2) has been developed.
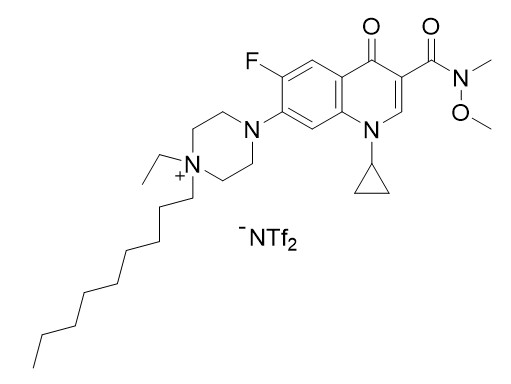
Figure 1: TSIL compound.
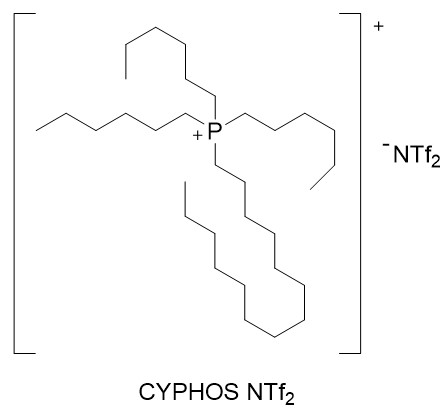
This extractant mixture is characterised by the fact that it allows the selective liquid-liquid extraction of f-block metals of the periodic table (lanthanide and actinide series) in samples containing other metals of the s-, p- and/or d-series.
The procedure to prepare the above TSIL compound is carried out in a very simple way from the antibiotic ciprofloxacin using conventional functional group transformations. The synthesis of this compound takes place in three steps under mild reaction conditions (room temperature to 0°C, atmospheric air pressure, etc.).
Step 1: N-alkylation reaction of ciprofloxacin with 1-bromoethane in the presence of excess N,N-diisopropyl(ethyl)amine to generate the following intermediate (see Figure 3) in 88% yield:

Figure 3: Reaction intermediate.
Step 2: Amidation reaction of the above compound using an excess of N,O-dimethylhydroxylamine in the presence of thionyl chloride to give the following Weinreb's amide (see Figure 4) in 86% yield:
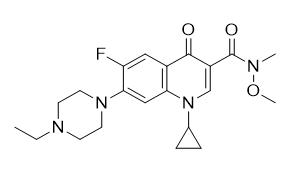
Figure 4: Weinreb's amide.
Stage 3: Alkylation reaction of the above compound by the addition of excess 1-iodonononane and subsequent ion exchange with lithium bis(trifuromethanesulfonyl)amidide to obtain the above TSIL molecule (see Figure 1) in 92% yield.
On the other hand, the procedure for the selective extraction of f-block metals in a sample containing f-block metals together with other s-, p- and/or d-series metals are as follows:
1) Prepare the extractant mixture (B) consisting of the compound TSIL from Figure 1 and the ionic liquid CYPHOS NTf2 from Figure 2.
2) Place the sample to be separated (A) containing one or more f-block metal(s) and other metals belonging to the s-, p- and/or d-series into a test tube. Adjust the pH to 6.
3) Add the extractant mixture (B) to the sample to be separated (A).
4) Shake for at least three minutes.
5) Wait until two liquid-liquid phases of different densities are differentiated.
6) Separate the two phases, the organic phase being the one corresponding to the extractant mixture (B) which includes the metal (or metals) of block f initially contained in the sample to be separated (A) (see Figure 5).
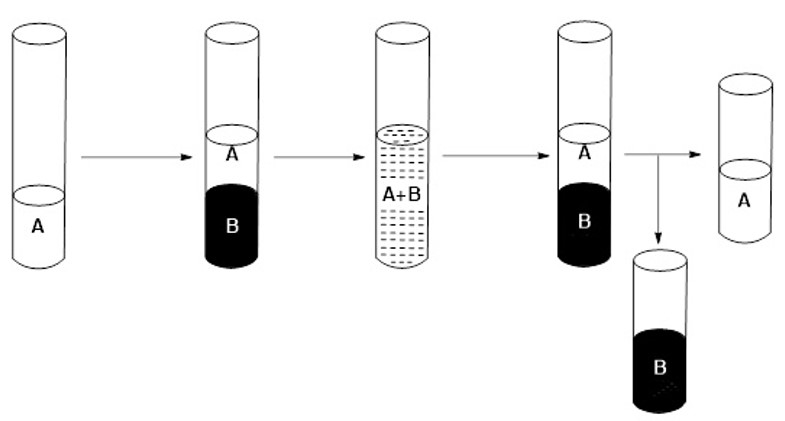
Figure 5: Schematic of the selective extraction process.
7) Add to the recovered organic phase (B) an acidified solution at pH=0.5 (C).
8) Stir.
9) Wait until two liquid-liquid phases of different densities are differentiated.
10) Separate the two phases, where the organic phase corresponds to the metal-free extractant mixture (B) and the aqueous phase (C) containing the metal(s) of f-block (see Figure 6).
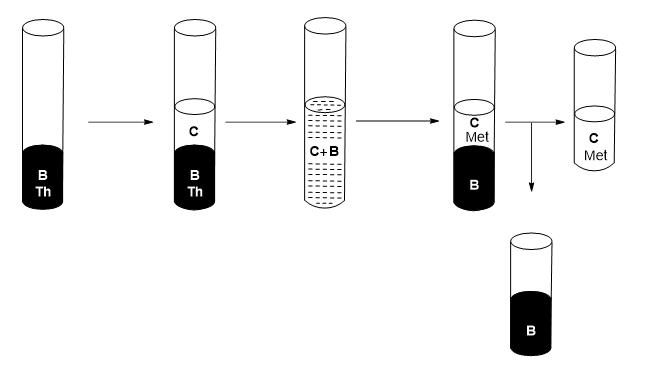
Figure 6: Schematic of the recovery process of the extractant mixture.
ADVANTAGES OF THE TECHNOLOGY
This novel extraction procedure has the following advantages:
1) It allows the selective extraction of inner transition metals (f-block) from metals belonging to the s-, d- and/or p-blocks of the periodic table in a very efficient way.
2) The extractant mixture is recyclable: once the extraction procedure has been completed, the complexed metal(s) can be fully recovered and the extractant mixture can be used in new extraction cycles.
3) The extractant mixture has a low affinity for the metals of the s-, d- and p- series of the periodic table, so that the metals of these series are extracted with a low or zero percentage.
4) The recovery rate of the extractant mixture is at least 95%, so it is possible to reuse it in new extraction cycles once the extracted metals have been released.
5) The extraction process is environmentally friendly.
6) The procedure is carried out under mild reaction conditions (temperature between 0°C-25°C and atmospheric pressure).
7) Both the TSIL compound and the CYPHOS NTf2 solvent are commercially available (or can be easily prepared by simple ion exchange).
8) The procedure is feasible on an industrial scale and can be adapted and implemented to the needs of the company.
In summary, the new extractant mixture is a revolutionary technology that significantly improves on current methods for the extraction of inner transition metals (lanthanides and actinides) in complex matrices.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The present extractant mixture [TSIL + CYPHOS NTf2] has several innovative aspects that differentiate it from other similar technologies on the market. In general, it is not common to combine TSIL compounds with ionic liquids.
Firstly, this novel chemical composition allows the selective and efficient extraction of the inner transition metals of the f-series (lanthanides and actinides) from other metals of the s-, d- and/or p-series of the periodic table. In this sense, the main interaction of the inner transition metals with TSIL, which acts as a selective chelator, takes place via the 1,3-dicarbonyl site.
Moreover, once the extracted metals have been separated, the original extractant mixture is recovered with a yield of more than 95%, which allows it to be recycled and subsequently used in new extraction cycles, making it a sustainable and environmentally friendly procedure (there is no other extraction system of these characteristics on the market that is recyclable).
The technology described has been developed on a laboratory scale (state of Technological Readiness Level: TRL = 3).
Tests have shown very promising results. A table with the analyte distribution coefficient (K), which is the ratio of the concentration of the metal in the organic phase to its concentration in the aqueous phase after the extraction procedure, is shown below (see Table 1).
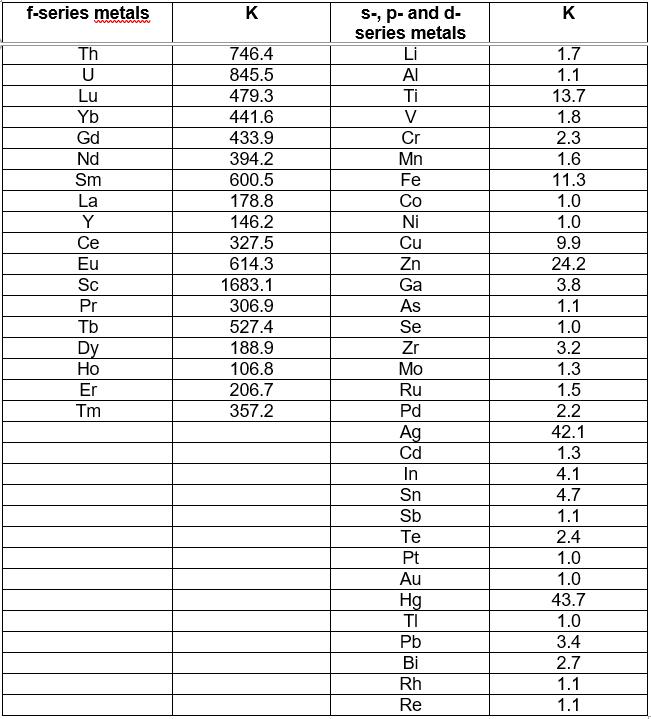
Table 1: The value of the distribution coefficients (K) obtained for the f-series metals, as well as for other metallic elements of the periodic table that can be found in their presence, at pH=6.
These tests have demonstrated the high efficiency and selectivity of the extractant mixture, since the higher the distribution coefficient of the analyte (K), the higher the extraction efficiency.
This novel composition is able to selectively extract the inner transition metals from the other metals of the periodic table at pH=6.
The main application sectors of this novel technology are:
• Mining.
• Nuclear chemistry.
• Nuclear medicine.
• Nuclear waste treatment.
• Scientific research.
This technology solves the problem of the selective separation of the chemical elements belonging to the rare earths (lanthanides and actinides), some of which are used as fuels in nuclear power plants.
The selective separation of these metals (f-block) from the rest of the metals listed in the periodic table is crucial both in the process of extracting the starting minerals and in the treatment of nuclear waste products.
Its application in different industrial sectors can have a positive impact on the environment and can contribute to improving energy sustainability worldwide.
Companies interested in acquiring this technology for commercial exploitation through patent licensing agreements are sought.
Company profile sought:
• Mining.
• Chemical industry.
• Nuclear industry.
• Nuclear medicine.
• Nuclear waste treatment.
The present invention is protected through patent application:
• Patent title: "Compuesto TSIL, procedimiento de preparación y procedimiento para la extracción selectiva de elementos de transición interna frente a metales de las series s, d y p empleando el compuesto TSIL ".
• Application number: P202330797.
• Application date: 23rd September 2023.
Geological and Geophysical Studies
Chemical Technology

Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




