Can we help you?
Contact us

Can we help you?
Contact us

Thank you for contacting us
Your form has been submitted successfully Our team will contact you again as soon as possible.
Whooppss...!! An error has occurred
Try sending later or write an email directly to areaempresas@ua.es

 PATENTED TECHNOLOGY
PATENTED TECHNOLOGY
INFO
SHEET
DOWNLOAD
EXECUTIVE
ABSTRACT
CONTACT DETAILS: Research Results Transfer Office-OTRI
University of Alicante
Tel.: +34 96 590 99 59
Email: areaempresas@ua.es
http://innoua.ua.es
The "Molecular Microbiology" research group at the University of Alicante has modified several phage proteins (Poll-N and UK-C) that have high specificity against Escherichia coli (E. coli), but not against other Gram-negative (G-) bacteria.
For these reasons, their use as a specific antimicrobial against E. coli is potentially interesting, especially in the case of contaminated food, cosmetics or water. It would also be useful in the treatment of diseases (infections) caused by E. coli.
The presence of a histidine tail attached to the phage proteins not only facilitates their purification but also improves lysis efficiency without the need of cell permeabilization treatments.
Companies interested in the commercial exploitation of the technology through license agreements and/or technical cooperation are sought.
Endolysins (along with holins) are one of the best-known protein families. Due to their ability to lysate bacteria, endolysins have been proposed as alternative to antibiotics, since the use of the latter has led to the emergence of resistances, considerably decreasing their effectiveness.
Additionally, the broad spectrum of action of many antibiotics can give rise to side effects during the treatment of infections, such as the alteration of the patient's natural microbiota by affecting different species in addition to the causative agent.
In the case of Gram-positive (G+) bacteria, the use of endolysins as an antibacterial agent is widely established. However, in the specific group of Gram-negative (G-) bacteria it has several limitations. Firstly, due to problems of accessibility to its target in the cell wall by the presence of an outer lipid membrane (OM). Secondly, there is very little variability in the cell wall composition of the different G- species, compromising specificity.
In order to alleviate the first problem, a permeabilizing OM treatment could be used, or tails of amino acids with net positive charge could be added to the endolysin to help overcome electrostatic repulsions due to the network of negative charges on the cell surface.
In order to solve the specificity issue, modular proteins can be used, with a catalytic domain and a cell wall-binding domain. However, the modular proteins described for G- are less numerous than for G+ and, in addition, their larger size hampers their purification and manipulation.
For the above reasons, derived from the hindrances that generally affect the use of endolysins in G-, there is a need to provide proteins with adequate lytic activity that do not require permeabilizing treatments, and that are specific to particular groups of bacteria.
The "Molecular Microbiology" research group at the University of Alicante has developed several modified phage proteins (Poll-N and UK-C) with antibacterial activity highly specific against E. coli without the need for previous permeabilization treatments.
Polypeptides (proteins) are a sequence of amino acids that are joined together by peptide bonds.
The polypeptides developed with endolysin activity, Poll-N and UK-C, comprise, respectively:
• An amino acid sequence according to SEQ ID NO: 3, or a derivative thereof (deletion, addition, insertion and/or substitution in this amino acid sequence), and a polycationic tail of amino acids (histidines) at the N-terminal end; and,
• An amino acid sequence according to SEQ ID NO: 4, or a derivative thereof (deletion, addition, insertion and/or substitution in this amino acid sequence), and a polycationic tail of amino acids (histidines) at the C-terminal end.
Once cloned, expressed and purified, the resulting proteins (Poll-N and UK-C) carry a histidine tail at their N-terminal or C-terminal end, being therefore different from the original. This tail not only facilitates its purification, but also favors the endolysin contact to the cell surface, thus improving its lysis efficiency.
The developed polypeptides can be used both as antimicrobial agents to prevent contamination by E. coli and in the treatment of diseases (infections) produced by E. coli.
The main advantages of the synthesized peptides, Poll-N and UK-C, are the following:
• The presence of a histidine tail at the N-terminal or C-terminal ends of synthesized peptides facilitates their purification and improves their lysis efficiency.
• They have a high specificity against E. coli as, among all bacteria tested, their lytic action is limited to those pertaining to this species without affecting any other belonging to other species, including closely related ones.
• Compared to antibiotics, a lesser probability of resistance occurrence is anticipated.
• Their use is expected to result, at most, in negligible effects on resident microbiota during the treatment of infections.
• Simple formulation, since no previous treatments of permeabilization of the external bacterial membrane are necessary, which in turn facilitates its application.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The main innovative aspect of the modified phage proteins is that they do not require prior treatments of OM permeabilization. Furthermore, the addition of nucleotides at the ends facilitates their manipulation and subsequent cloning in the appropriate expression vector. Finally, another innovative aspect is the generation of anti-E. coli endolysins whose sequence is significantly different from others.
The technology is developed at laboratory scale.
The efficacies of purified Poll-N and UK-C endolysins have been tested by means of spot test experiments against different bacterial strains. Figure 1 (for Poll-N) and Figure 2 (for UK-C) show the appearance of growth inhibition zones produced by lysis.
The results obtained show that both Poll-N and UK-C are capable of directly lysing the majority (92.5% for Poll-N and 91.2% for UK-C) of E. coli strains tested (159 in total).
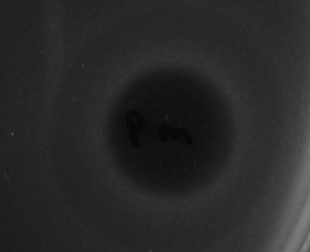
Figure 1. Result of a spot test experiment carried out with Poll-N at a concentration of 16 µg/mL on an E. coli strain. Bacterial growth is visualized as a gray layer on the plate. The dark central area, where the protein solution was loaded, indicates the inhibitory effect in growth produced by the endolysin.
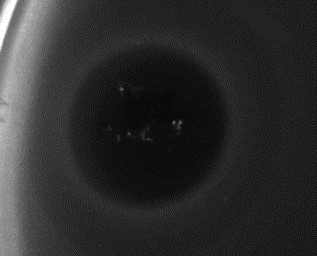
Figure 2. Result of a spot test experiment carried out with UK-C at a concentration of 16 µg/mL on an E. coli strain. Bacterial growth is visualized as a gray layer on the plate. The dark central area, where the protein solution was loaded, indicates the inhibitory effect in growth produced by the endolysin.
The present invention is framed in the general field of genetic engineering and, in particular, it refers to viral proteins that have been modified by means of the addition of a polycationic tail of amino acids at the N-terminal or C-terminal end, in such a way that they present antibacterial activity against E. coli without previous treatments of envelope permeabilization. Both proteins show a high specificity to E. coli.
Therefore, the developed polypeptides can be used both as antimicrobial agents against E. coli (particularly in food, cosmetics, water contaminated with E. coli, etc.), as well as in the treatment of diseases (infections) produced by E. coli.
This technology could be applied in biosanitary, veterinary, biotechnological, or agri-food companies interested in antimicrobial treatments alternative to antibiotics to control the growth of E. coli.
Companies interested in acquiring this technology for its commercial exploitation through technology transfer agreements (see below) are sought:
• Patent licensing agreements.
• Technical cooperation agreements (R&D projects) for the use of the technology or application in other sectors.
• Subcontracting agreements for technical assistance, training, etc.
Type of company sought:
• Companies in the biotechnology sector.
• Companies in the pharmacological sector.
This technology is protected by patent application.
- Title of the patent: "Viral proteins with antibacterial activity against E. coli".
- Application number: P201930890.
- Date of application: 10 October 2019.
Molecular Biology and Biotechnology
Pharmacology, Cosmetics and Ophthalmology
Medicine and Health

Carretera San Vicente del Raspeig s/n - 03690 San Vicente del Raspeig - Alicante
Tel.: (+34) 965 90 9959




